| |
Importance of Anionic Reactive Intermediates for Lubricant Component Reactions with Friction Surfaces
Czes³aw Kajdas,
Technical University of Radom, Poland
Keywords: electron emission, anions, chemisorption, protective film, boundary lubrication
Published in: Lubrication Science 6, 3, April 1994, p.203.
Abstract
To understand the chemical behaviour of lubricant components during boundary lubrication, a general concept of negative ionradical reactive intermediates formation for these components has been proposed. The concept is based upon the ionisation mechanism of these compounds caused by the action of electrons of law energy (1-4 eV). Electrons of such energy (exoelectrons) are spontaneously emitted from most fresh surfaces formed during friction. The principal thesis of the model is that lubricant components form anions which are then chemisorbed on the positively charged areas of rubbing surfaces.
The formation of negative ions and their decomposition process are stimulated by electron attachment mass spectrography. This type of mass spectrography uses electrons of an energy range similar to those of exoelectrons. The proposed model encompasses the following major stages: (a) low-energy electron emission process and creation of positively charged spots, (b) action of emitted electrons with lubricant components (anion and radical formation), (c) reactions of anions with metal surfaces forming a film protecting the surface from wear, (d) cracking of chemical bonds producing other radicals. The model explains many lubrication phenomena of hydrocarbons, oxygen-containing compounds, and many types of chemicals used as antiwear and extreme-pressure additives.
INTRODUCTION
The most important group of lubricants includes lubricating oils, formulated base fluids, and additives. The base fluids include petroleum hydrocarbons and/or synthetic materials. The additives encompass many chemicals, especially polar ones. Organic compounds containing such elements as S, O, Cl, P, N, as well as organometallic compounds, are the commonly used boundary lubrication additives.
In boundary lubrication solid surfaces are so close together that appreciable contact between opposing asperities is possible. The friction and wear in boundary lubrication are determined predominantly by interaction between the solid and the liquid [1]. Thus, the chemistry of friction surfaces and their state determine their interaction with the lubricant components.
The ability of a lubricating oil to reduce wear and prevent damage of interacting solids is the crucial factor controlling lubricant formulation. Therefore, the chemistry of surfaces under a given set of boundary friction conditions and the chemistry of the lubricant components, i.e., the chemical reactivity of the system, control friction and wear. Chemical reactions of lubricant components, especially of antiwear and extreme-pressure additives, occurring during a tribological process involve the formation of a film on a contact surface, to alter the surface's character and so protect it during friction.
Chemical reactions with tribological solid surfaces may be initiated by temperature and impact. It is well known that friction produces local high temperatures. In addition to the temperature effects at surface boundaries when two solids rub together, there is the enhanced surface activity caused by the friction process. The enhanced surface activity might be expressed as follows [2]:
Enhanced reactivity = exoelectron + catalytic factors + elevated temperature + high pressure
The mechanical action at solid surfaces tends to promote chemical reactions and produce surface chemistry that may be entirely different to that observed in static studies. Under boundary friction conditions, the exposed surface is extremely reactive due to mechanical activity - particularly in the case of metals and alloys. Hydrocarbons can chemisorb and even chemically react with the metal surface [3]. In other words, friction initiates and accelerates chemical reactions that otherwise would take place at much higher temperatures or will not initiate. These facts have long been observed, but explanations are still controversial [4]. These phenomena make up a special branch of chemistry dealing with the chemical and physico-chemical changes of solids due to the influence of mechanical energy [5].
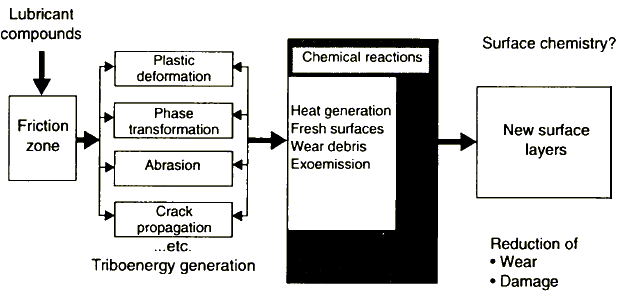 | |
Figure 1 Physical processes associated with surface enlargement during boundary friction
|
BACKGROUND
The action mechanism of the main lubricating oil components, especially antiwear and extreme-pressure additives, is complex and, so far, it has not been generally explained from the viewpoint of chemical transformations. During friction, as a result of elevated temperature and oxidation, the mechanism of a number of transformations of lubricating oil components involves free radicals. However, the situation appears to be much more complicated.
Generally, the application of mechanical energy associated with friction releases a great number of physical processes (Figure 1) which can be the cause of tribochemical reactions of solids with molecules of lubricants. Apart from electrons, other particle types [6], e.g., ions, lattice components, might be emitted from solids during-mechanical deformation. The most important are electrons.
The reaction of the emitted electrons with lubricating oil molecules adsorbed on the friction surface may lead to the formation of negative ions and radicals [7]. Thus, the negative ion-radical lubrication mechanism can be taken into account as well.
Before presenting some details concerning the emission of exoelectrons during friction, it is convenient to discuss selected problems associated with the influence of temperature on tribochemical reactions. In comparison with the thermally activated reactions, an altered temperature dependence has been found in tribochemical reactions [5, 8, 9]. Numerous tribochemical reactions are independent of temperature in a wide (0-150°C) range. It appears that the activation energies always have smaller values with tribochemical treatment than with exclusively thermal stress. The occurrence of a reaction velocity independent of temperature shows clearly that the energy needed for releasing the reactions is applied to the solid through the mechanically activated regions only. It was found [9] that the initiation of tribochemical reactions of aluminium with alkyl halides was not due to the temperature rise at mechanical contact but to the active sources formed on an aluminium surface by vibromilling. The reactivity of milled aluminium was well correlated with the intensity of the exoelectron emission [9].
In other work [10] it was found that the temperatures determined from the reactivity of tribological surfaces were much higher than those calculated by the equation [11]. For example, for lower loads, differences of over 200°C were noted. This finding is in agreement with the above tribochemical factors.
The term 'exoelectron emission' [12] (Kramer effect) originates from investigation of the emission in freshly treated metals which were accounted for as a consequence of exothermal transformation processes of the surface. Exoelectrons are electrons of low energy spontaneously emitted from most fresh surfaces. At present, the term exoelectron emission is used as an inclusive term for emission phenomena which proceed unsteadily, with changed work function, and after excitation by non-thermal energy.
Materials such as graphite, molybdenum disulphide, and polytetrafluoroethylene emit few or no electrons when disturbed, whereas fresh surfaces of metals, such as aluminium and steel, produce large numbers of these electrons [13]. Exoelectron emission occurs when a material's surface is disturbed by plastic deformation, abrasion, fatigue cracking, and phase deformation. The electron emission from freshly formed surfaces reach a maximum immediately, then decays with time. Figure 2 depicts that the current of negatively charged particles emitted from oxide-covered aluminium consists of both electrons and negative ions. Some emission of positive ions and photons was detected as well, however, at the strain rate of 2.2 x 10 -4 sec -1, the emitted particle flux was weak [6]. Electron emission has been observed for both metals and non-metals, and there is strong evidence that the existence of oxides or other non-metallic surface layers is necessary for electron emission.
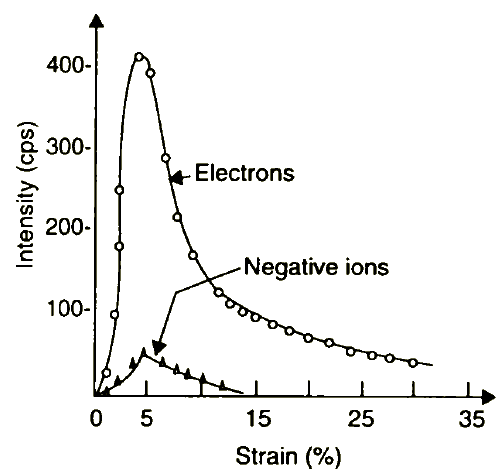 | |
Figure 2 Tribostimulated emission rates of electrons and negative ions v strain obtained from aluminium covered with 2000 Å thick dense aluminium oxide. Deformation rate •e = 2.2 x 10 -1 sec -1
|
Exoelectron emission occurs from a clean, strain-free surface upon adsorption of oxygen [14]. Since exoelectrons have a kinetic energy of about 1-4 eV, [5, 6, 15] they may involve some chemical reactions. However, only limited investigations [9, 16, 18] have been concerned with the chemical effect of exoelectrons.
Probably the first work pertinent to tribochemistry was performed by Shaw [19]. Grunberg, [17], succeeded in showing that the metal-cutting process under water can produce hydrogen peroxide. He explained his results in terms of the Kramer effect. Goldblatt [20] explained the lubricating properties of polynuclear aromatics by assuming that, as a first step in the chemical processes, radical anions of the aromatic hydrocarbons are generated at the freshly abraded surface. He assumed that one of the first processes taking place under boundary lubrication conditions is the electron transfer process. Rosenfeld [21] reported hydrogen formation in some lubricating oils during sliding wear experiments. He concluded that hydrogen formation is the result of a chemical reaction which occurs between the lubricating oils and the fresh metal surfaces.
PRESENT WORK
This paper describes a general anionic-radical lubrication model based upon the ionisation mechanism of lubricating oil components caused by the action of low energy (1-4 eV) electrons. Electrons of an average energy of 3 eV are used in the mass spectrography of negative ions [22]. The energy of these electrons seems to be close to the energy of exoelectrons emitted from freshly formed surfaces during the friction of metal surfaces. Thus, the simulation of ionisation and fragmentation processes of lubricating oil components can be based on this technique [22].
The most important chemical processes are chemisorption of active components on a metallic (or ceramic) surface and degradation of organic or organometallic molecules. For most additive reactions taking place on surfaces, the sequence of events is so complex that reactive intermediates are not usually involved in the reaction mechanism.
Two basic factors arise in assessing the role of reactive intermediates of antiwear and extreme-pressure additives in the formation of load-carrying layers by tribochemical reactions:
- mechanism of reactive intermediates formation, and
- mechanism of their degradation.
The basic aim of this paper is to present details connected with these factors.
GENERAL MODEL
The general model of the negative ion-radical action mechanism of lubricating oil components assumes creation of two types of activated sites on friction surfaces, i.e., thermally activated sites and sites activated by the exoelectron emission (EEE) process. The situation is illustrated in Figure 3.
In comparison with a thermally stressed solid, mechanically treated solids show a reactivity often increased by several orders of magnitude, particularly in the low temperature range. For tribochemical reactions, only limited use can be made of the relationship of classical thermodynamics; thus, reactions with a positive free enthalpy also appear during mechanical treatment [5]. High-energy wear particles are also important.
This author believes that the most important factor governing the tribochemical reactions under boundary friction is
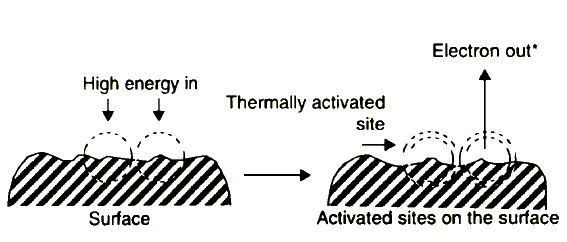 | |
Figure 3 Surface activation during friction |
* One of the factors controlling EEE is the work function, f, of a solid
|
associated with the action of exoelectrons with lubricating oil components.
Therefore, lubricant components acting with exoelectrons form negative ions which are then chemisorbed on the positively charged areas of friction surfaces. The following states have been considered:
1. Low energy electron (1-4 eV) emission process (exoemission) and creation of positively charged spots, generally on top of asperities (Figure 4). The EEE process depends on friction conditions, e.g., type of material, load, and atmosphere. The atmosphere of the tribological system is of particular importance as two components of principal significance for boundary lubrication are oxygen and water vapour.
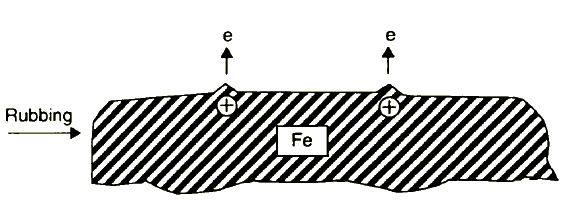 | |
Figure 4 Simplified presentation of EEE process during boundary friction
|
Action of the emitted electrons with lubricating oil component molecules - when close to the contact area - causing the formation of negative ions and radicals.
It is known from the mass spectrometry technique that if the energy of electrons is sufficiently high (usually greater than 10 eV) the impact of electrons with a molecule AB yields a positive ion in the reaction
AB + e → AB+ + 2e
which may or may not dissociate to give rise to fragment ions.
The energy of exoelectrons is much smaller and it is not sufficient to produce positive ions. In the conditions of electron attachment mass spectrography, negative ion spectra can be obtained. Electrons of an average energy of about 3 eV [22] are attached to the molecules and negative ions are formed according to the mechanisms presented in Figure 5.
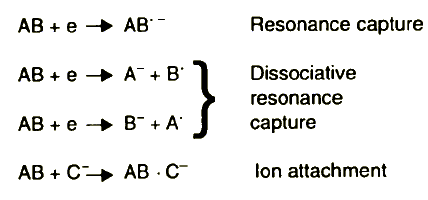 | |
Figure 5 Negative ion formation mechanisms in electron attachment mass spectrograph
|
It is assumed that the exoelectron energy is sufficient to cause the ionisation of lubricating oil component molecules and the conditions in friction microareas approximate those existing in the plasmatic electron source of the electron attachment mass spectrograph. This is very probably due to the low energy of electrons produced in the ion source, which amounts on average to about 3 eV. Thus, reactions of various compounds that take place in the ion source of this mass spectrograph may be roughly compared with reactions taking place in the friction zone. The number of ions being formed, and their kind, depend on the chemical character of the compound. The difference in negative ion formation of various compounds can be explained in terms of the difference in their electron affinity.
The proposed approach to simulation of tribochemical reactions in the plasmatic ion source of the electron attachment mass spectrograph might be supported by the magma-plasma model [24] elaborated by Thiessen (Figure 6). According to this, the highest energy states, called triboplasmas, appear immediately during the impact.
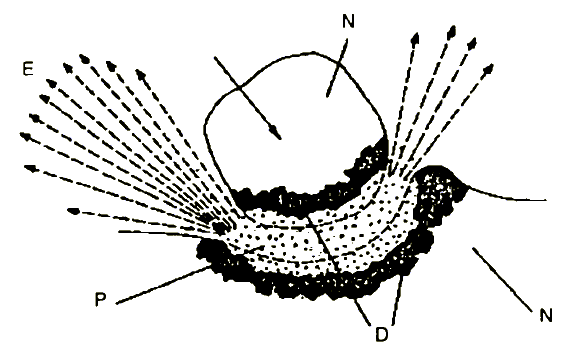 | |
Figure 6 Magma-plasma model for the impact stress of flying grain |
E - exoemission
N - normal structure
P - plasma
D - disordered structure
|
They are characterised by an extremely disturbed solid structure as well as by non-steady highly excited fragments of the solid and of the surrounding chemical reactions sphere in the form of lattice components.
The short life of the triboplasma causes no Maxwell-Boltzmann distribution so that the equilibrium temperature cannot be given and the chemical process taking place in this excitation phase cannot be described by the laws of thermodynamics [24]. The conversions in triboplasmas are of a stochastic nature.
3. Reaction of negative ions with metal (or ceramic) surface (chemisorption) and other reactions, e.g., free radical reactions, forming an organometallic (or inorganic) film protecting the rubbing surfaces from wear (Figure 7).
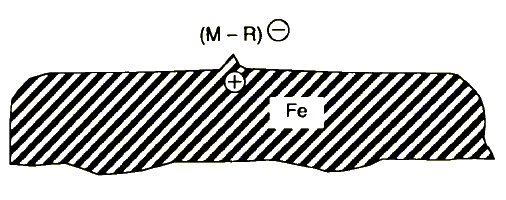 | |
Figure 7 Chemisorption of negative ions (M - R)- formed in stage 2: M + e → (M-R)- + R°, where M is the lubricating oil component molecule
|
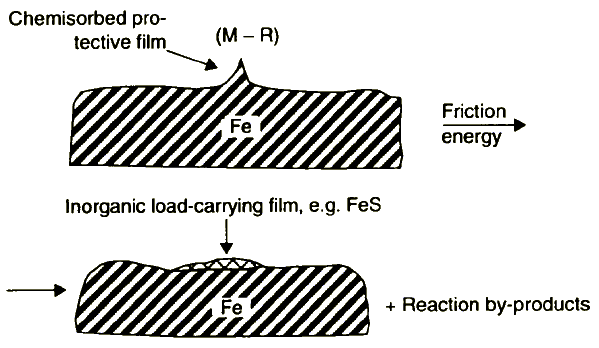 | |
Figure 8 Schematic representation of load-carrying film formation under extreme pressure friction conditions
|
The kinetics of such reactions depend on the susceptibility of the lubricant molecule to negative ion formation. The free radicals (stage 2) may form from friction resins, and in the presence of oxygen they undergo oxidation:
R° + O2 → ROO°
ROO° + RH → ROOH + R°
ROOH → RO° + HO° etc.
4. If shear strength is high (extreme-pressure friction conditions) it can cause cracking of chemical bonds producing inorganic load-carrying film and reaction by-products (Figure 8).
This process is controlled by the bond strength of a given lubricant molecule. Usually, the load-carrying ability of the film formed increases as the ease of scission of the closest bond to the element chemisorbed on the surface decreases.
5. Destruction of an inorganic soft layer - protecting surface from seizure - connected with the creation of high temperature spots, electron emission and high energetic wear debris formation processes (Figure 9).
The activated surface spots, shown in Figure 9, form a new protective film according to stages 2 to 4. Figure 10 summarises the essential features of this lubrication model.
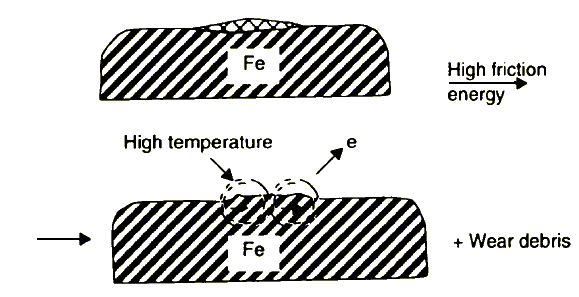 | |
Figure 9 Destruction of the load-carrying film and creation of activated surface spots
|
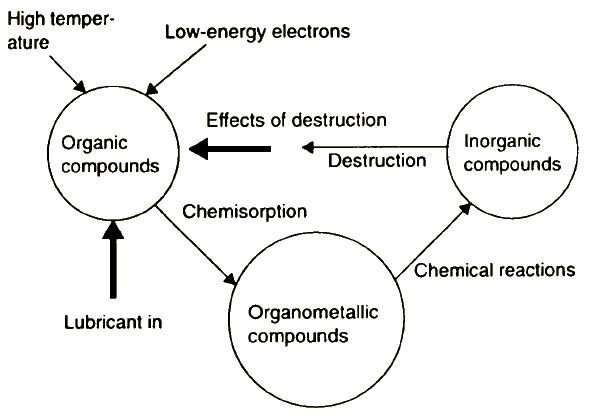 | |
Figure 10 Reaction cycle of lubricant components on solid contacts during friction
|
REACTIVE INTERMEDIATES:
Ionisation and fragmentation mechanisms of lubricating oil components
To account for the behaviour of lubricant molecules during friction, it is necessary to have data on their reactive intermediates. These may be obtained using the negative-ion mass spectrograph. The greater part of the data used in this paper was taken from reference 22, that describes ionisation and fragmentation features of various classes of organic compounds; the measurements for hydrocarbons and organometallics have been carried out using the equipment described therein. The energy of bombarding electrons was 1-4 eV, temperature of the ion source 35-50°C. The formed ions were recorded on photographic plates. A photometer was used to transform the lines from the plates to mass spectra. The application of negative-ion mass spectra to elucidate some details concerning reaction mechanisms of the most important lubricating oil components is discussed below.
Hydrocarbons
Most frequently, negative ions are produced according to the mechanism:
|
M + e — |
┌→ Mo—
│
└ → (M-1)¯ + M° |
Saturated hydrocarbons of any structure are an exception to this. To obtain negative ions of this type of molecule, OH¯ ions have to be attached:
M + OH¯ → M.OH¯
This mechanism of ionisation could be tested with small quantities of heavy water vapour in the ion source of the electron attachment mass spectrograph. Because of the attachment of OD¯ ions, the peaks are shifted to the next highest mass number as shown for n-hexatriacontane in Figure 11.
The aromatic hydrocarbons can yield two types of negative ions, e.g., typical electron attachment.
M + e → Mo—
which is especially characteristic of condensed polycyclie aromatics, and attachment O¯ :
M+O¯ → (M - 1)Oo— + M°
Appeldoorn and Tao [26], and Goldblatt [20], showed that under boundary lubrication the presence of dry argon produces the highest wear in the presence of L- and B-methylnaphthalene and idene. When used as lubricating agents in air, these hydrocarbons clearly counteract the wear. Saturated hydrocarbons, monoaromatic naphthenes (e.g., phenylcyclohexene) and diphenylmethane behave in a quite different way (Table 1).
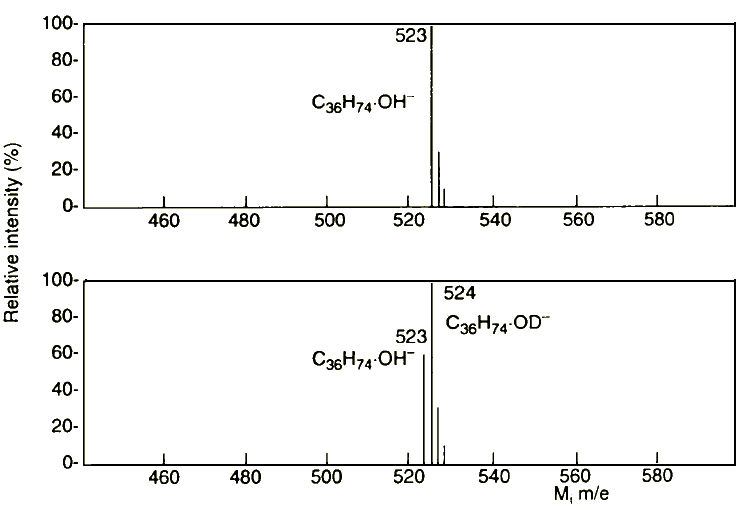 | |
Figure 11 Attachment of OH¯ or OD¯ to n-hexatriacontane in the presence of H2O and D2O vapour on the ion surface (from reference 25)
|
The difference between the tribological properties of L-methylnaphthalene (wear in argon, 0.82 mm; wear in air, 0.33 mm) and diphenylmethane (wear in argon, 0.29 mm; wear in air, 0.43 mm) is particularly worth noting. This inconsistent behaviour of aromatic and saturated hydrocarbons in different atmospheres under boundary friction can be explained by the proposed model of the. formation of the reactive intermediates.
The better performance of condensed aromatics in air than in argon can be explained as follows. Condensed aromatics in argon ionise according to the electron capture mechanism. The radical ions reacting with positively charged spots on the friction surfaces lead to accelerated wear, similar to that in reference 20, described by the reactions:
M°- + metal or metal oxide → reduced metal oxides or abrasives
abrasives + metal → abrasive wear
Table 1
Effect of two-ring hydrocarbons on wear in different atmospheres [26] | |
| Wear scar diameter* (mm) in atmospheres|
Hydrocarbon | Dry argon | Dry air | Wet air|
Decalin | 0.26 | 0.35 | 0.42 | |
Phenylcyclohexane | 0.31 | 0.33 | 0.50 | |
Tetralin | 0.25 | 0.42 | 0.68 | |
Indane | 0.30 | 0.31 | 0.42 | |
Diphenylmethane | 0.29 | 0.43 | 0.62 | |
Methylnaphthalene | 0.82 | 0.33 | 0.36 | |
Indene | 0.93 | 0.72 | 0.33 | |
*Ball-on-cylinder tests; load, 9.81 N; temperature, 25°C; 240 r/min; work period, 32 min.
| | |
In the presence of water or oxygen, ions form by another mechanism. These radical ions, (M - 1)Oo—, react with positively charged spots to control wear, causing a substantial decrease in wear. According to the model presented in reference 20, quenching of radical anions Mo— by either water vapour or oxygen accounts for the lower wear relative to inert atmospheres.
Saturated hydrocarbons tested performed better in dry argon than in the presence of water or oxygen. This is because under such (dry) conditions negative ions cannot be formed. Thus the hydrocarbons can properly perform their function as lubricants (no corrosive wear can occur). If water or oxygen is present, OH¯ ions are produced that, in turn, react with saturated hydrocarbon molecules to form M:OM¯ ions. These ions would show behaviour similar to that of aromatic (M - 1)O¯ ions. This is corroborated by the general similarity between wear with monoaromatics and that with saturated compounds tested in the presence of water or oxygen (Table 1). The ions (M-1)O° and M:OH¯ of all these compounds contain oxygen, which would cause similar interaction of the negative ions with the positively charged spots of rubbing surfaces.
Jahanmir and Fischer [27] have given clear evidence that in boundary lubrication of silicone nitride there should be chemical interaction between n-hexadecane and the ceramic surface.
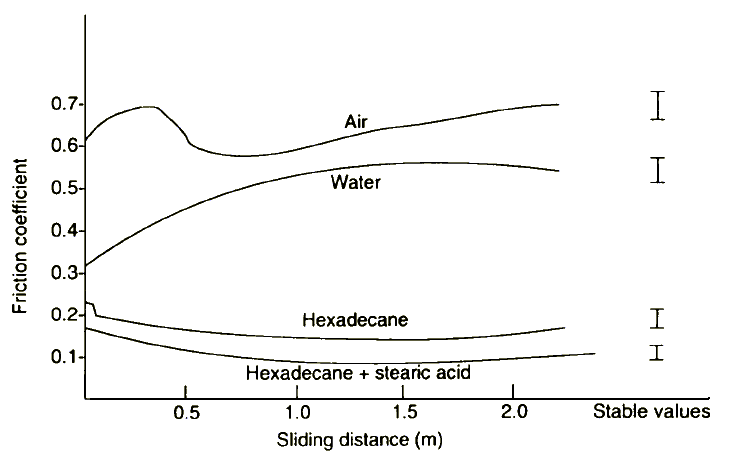 | |
Figure 12 Friction coefficient of Si3N4 sliding against itself at low speeds (1 mm/sec) in various environments plotted as a function of sliding distance; load was 9.81 N (from reference 27)
|
Three points were very important:
- tribochemical reaction of n-hexadecane does not form Si-C bonding
- tribochemical reaction of n-hexadecane transfers oxygen to the sliding surface; and
- reactive intermediate molecules form a boundary film- resulting in a very significant decrease of friction coefficient (Figure 12).
This finding can be explained in terms of the negative ion-radical model as well. Thereafter, n-C16H34.OH¯ ions can react with the positively charged surface spots forming a chemisorbed film that decreases friction coefficient and wear. Any destruction of the film during sliding should lead to the formation of SiO2 and of some amorphous products containin.ig Si, C, N, and O, as shown by experimental data.
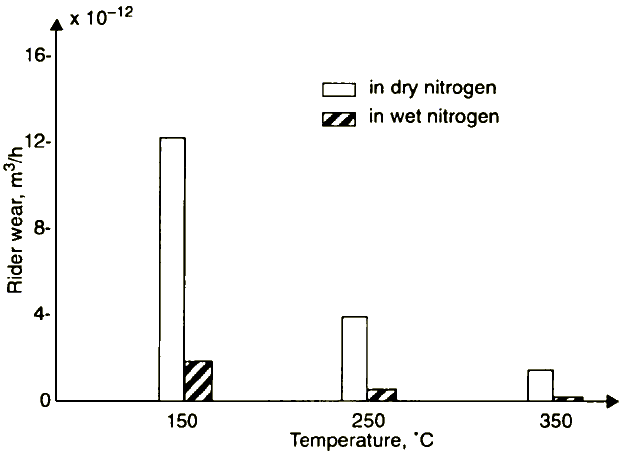 | |
Figure 13 Rider wear for phenyl ether v temperature in wet and dry nitrogen (from reference 28)
|
Polyphenyl ether
Jones and Hady [28], investigating polyphenyl ether under boundary lubrication of steel, found that substantially lower wear occurred in wet nitrogen compared to dry nitrogen (Figure 13). Figure 13 also depicts that increasing temperature decreases the wear significantly, especially in dry nitrogen. These findings can be explained in terms of the proposed lubrication model. In dry argon and at lower temperature, polyphenyl ether reacting with low-energy electrons yielded (M-1)¯ ions. These ions react with positively charged spots forming Fe-C bonding, which leads to accelerated wear; for example, FeC debris may be produced.
Water molecules interacting with electrons produce OH¯ ions that, reacting with the surface positive charged spots, control wear by formation of a protective oxide layer. The quenching of (M-1)¯ ions by water molecules
(M-1)¯- + H2O → M + OH¯
is also conceivable.
Elevated temperatures cause fragmentation of (M-1)¯ ions, yielding (Ph-O)¯ ions where Ph is the phenyl group and n = 1 to 4, i.e., Ph-O¯, Ph—O—(PH-1)O¯, etc. These ions oxidise the surfaces, thus reducing the wear. The higher the concentration of (Ph-O)¯ ions, the better the antiwear effect.
Morales [29] found that benzene forms polyphenyl ether-type products during boundary lubrication conditions. To elucidate the mechanism by which benzene forms these products, he used organic electrochemistry. His results indicate that an electron transfer process occurs, leading to the formation of polyphenyl either-type products by means of an intermediate. Phenol formation is proposed as follows [29] :
HO° + benzene (PhH) → phenol (PhOH) + H°
Using the negative ion-radical lubrication mechanism, an alternative approach based on (M-1)¯ may be proposed. PhO¯ ion should be the appropriate reactive intermediate leading to the formation of polyphenyl ether-type products.
Alcohols
The basic decomposition process of higher-molecular weight fatty alcohols is the splitting off of three hydrogen atoms
(R—CH2—OH)o— → R—•C•—O¯ + 3H°
However, (M-2)¯, (M-2)o—, and (M-5)¯ anions are present in comparatively large amounts (Table 2).
Table 2
Type and intensity of fatty alcohol negative ions (from reference 22) | |
| Ion intensity (%)|
Fatty alcohol | M¯ | (M-1)¯ | (M-2)o— | (M-3)¯ | (M-5)¯|
n-C8H17OH | 7.0 | 50.0 | 25.0 | 100 | 10.0 | |
n-C9H19OH | 5.8 | 50.0 | 9.0 | 100 | 7.2 | |
n-C12H25OH | 5.7 | 46.0 | 16.2 | 100 | 5.1 | |
n-C14H29OH | 5.0 | 40.7 | 18.1 | 100 | 5.0 | |
n-C16H33OH | -8.7 | 50.6 | 16.5 | 100 | 5.7 | |
n-C18H37OH | 10.0 | 45.0 | 23.8 | 100 | 6.5
| | |
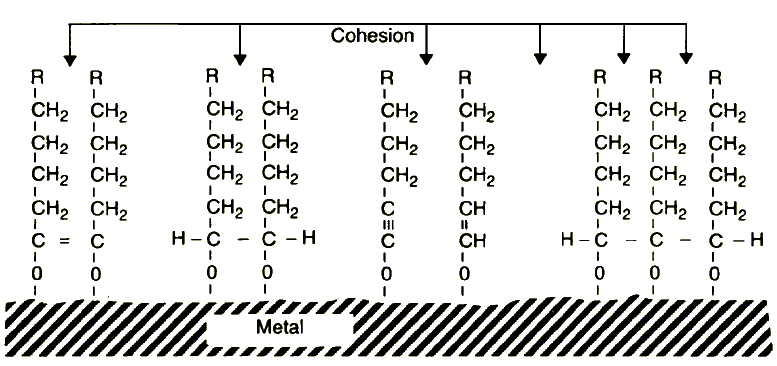 | |
Figure 14 Model of fatty acohol chemisorption during friction of metals
|
The structure of these ions can be presented at follows:
(M-1)° = R—CH2—CH2—O¯
(M-2)° = R—•CH2—CH—O¯
(M-2)° ≡ R—C═CH—O¯
Low molecular weight alcohols are an exception. The only decomposition process of methyl alcohol is the splitting-off of one hydrogen atom. The intensity of the (M-3)¯ ion in the ethyl alcohol mass spectrum is very low. It increases with the increasing chain length of the alcohols, reaching n-hexyl alcohol values comparable with those given in Table 2.
Taking into account the specificity of the decomposition process of fatty alcohols, one can propose a model of chemisorption caused by friction of these compounds (Figure 14).
As shown by reference 30, the wear of aluminium surfaces lubricated with normal C8÷C18 alcohols was largely chemical; there was no mechanical disturbance of the aluminium surface at all and the metal structure was unchanged right up to the surface. The infrared spectra of a protective film of waxy appearance, removed from the aluminium pin, indicated that the material was crystalline and possessed a carbon-oxygen-metal grouping and an alkyl chain. It was concluded that alcohols react with the aluminium surface when sliding occurs. No explanation for the reaction mechanism was given.
Now this finding can be accounted for in terms of the proposed model of the lubrication mechanism of alcohols towards metal emitting exoelectrons during sliding. As can be seen in Figure 14, metallo-organic compounds desorbed from an aluminium surface during friction should possess the carbon-oxygen-aluminium grouping and an alkyl chain. Alternatively, high energy aluminium wear debris, able to emit exoelectrons, can react directly with alcohol molecules, forming different types of compounds which always possess the carbon-oxygen-metal grouping and an alkyl chain.
The model is corroborated by work [31] showing that the pentaerythritol partial ester, which contains both hydroxyl and ester groups, reacts with the surface aluminium atoms to form amorphous substances, such as aluminium complexes. These wear tests were performed on a pin (aluminium)-on-disk (steel) friction machine, the wear scar produced in the partial-ester solution was very smooth, and some of the wear particles formed a stable dispersion in the base oil because of their small size, and could not be separated from the base oil by centrifuging. It is not clear from the results of the study [31] what type of aluminium complex or salt was formed on the rubbing surface of the aluminium; it was only concluded that the partial ester reacts with surface aluminium atoms to form an amorphous aluminium complex or salt after chemisorption of the partial ester on the aluminium surface. This reaction product was a very effective antiwear agent for aluminium.
Explaining this finding in terms of the proposed lubricating mechanism of alcohols, it can be presumed that the wear products are composed of aluminium complexes having big ligands attached to the metal through the hydroxyl oxygen.
Another study [32] found that 2% n-hexadecanol in n-hexadecane produced an adherent coating on the sliding steel surfaces, which was composed of many compounds, including alkynes, dialkynes, alkenes, and dialkenes with a maximal molecular weight rise of about 100%. By using 1% hexadecane-1, 16-diol in n-hexadecane as the lubricant, many compounds, similar to those formed from n-hexadecanol were found in the coating on the sliding surfaces. In this case the maximal molecular weight rise was about 50%. Under these conditions, pure alkanes, and 2% stearic acid in n-hexadecane, did not form adherent layers, which might be referred to as friction resin.
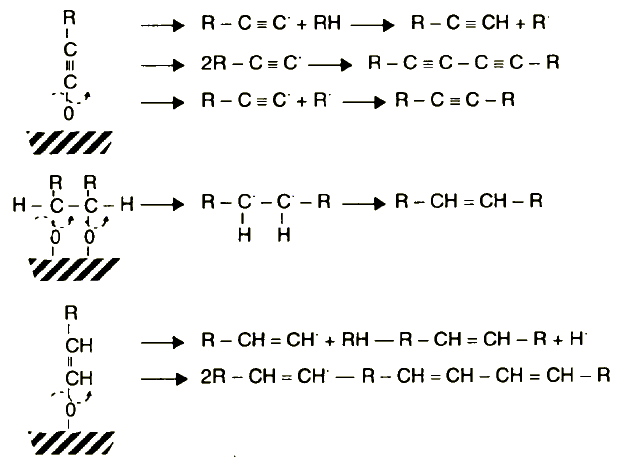 | |
Figure 15 Schematic representation of alkyne, dialkyne, alkane, and dialkene formation by different types of chemisorbed anions produced from alcohols
|
These tests were deliberately carried out on very pure fluids in order to ascertain specifically the behaviour of various chemical functional groups.
The formation of alkynes, dialkynes, alkenes, and dialkenes explains stage 4 (cracking of chemical bonds of chemisorbed alcohols) of the proposed model (Figure 15). Further unidentified, higher molecular hydrocarbon components of the coating produced from n-hexadecanol might be derived by-the destruction of the cross-linked alcohol film (Figure 14).
Furthermore, the model elucidates the finding of Mori, Suginoya, and Tamai [33] of the formation of aluminium methoxide and aluminium butoxide on an aluminium surface prepared by cutting under high vacuum; These experiments were conducted in a special vacuunTchamber. A small type of lathe was constructed in the chamber. The aluminium disk was mounted on a drive shaft which was rotated using a magnetic assembly.
Reactants were introduced to the chamber through a variable leak valve. The components in the chamber were analysed by a mass spectrometer. It was stated that the rate of chemisorption was proportional to the cutting speed and the chemisorption took place not only during but also after cutting. The hydrogen concentration increased during cutting.
The reaction mechanism of this finding can be explained in terms of the negative ion-radical model as follows. The cutting process is associated with the exoelectron emission that is responsible for the rate of chemisorption. Thus, the exoemission process is the main factor governing the reactivity of methanol and butanol towards aluminium. It is known that exoelectron emission during rubbing reaches a maximum immediately, then, after rubbing, decays with time; it thus explains the fact that chemisorption took place after cutting. Hydrogen radical H° recombination is responsible for the hydrogen formation during cutting.
Organosulphur compounds
It has been generally accepted that the extreme pressure performance of disulphides is better than that of monosulphides [34, 35]. The difference can be explained simply with the negative ion-lubrication model. Monosulphides and disulphides ionise according to the dissociative capture mechanism. The basic process of sulphides decomposition is the cleavage of the C-S bond
R—S—R + e → RS¯ + R°
and, in the case of disulphides, decomposition S—S bond cleavage
R—S—S—R + e → RS¯ + RS°
where RS°+e' → RS¯
where e' is an electron of lower energy.
Thus, it has been concluded that the disulphides will exhibit more efficient load-carrying properties due to the fact that lower energy is needed for the formation of the same number of RS¯ ions.
The chemical structure of sulphides and disulphides has a marked effect on performance. For example, the extreme-pressure performance of dibenzyl sulphide was much better than that of diphenyl sulphide [35, 36].
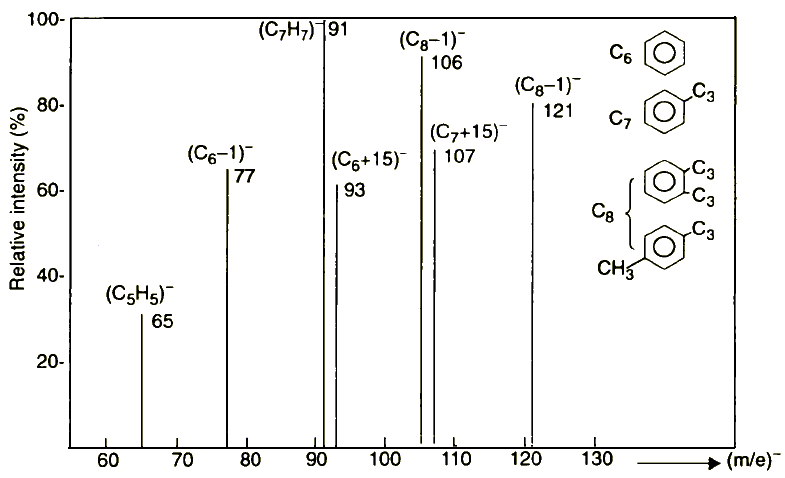 | |
Figure 16 Electron Attachment Mass Spectrum (without isotopic ions) of a mixture of C6÷C8 aromatic hydrocarbons (benzene tolyene, p-xylene, and o-xylene in ratio 1:1:1:1; chemical API quant. Kit no. 25-A; energy of electrons 2-4 eV; temperature 50°C)
|
This difference can be explained as follows. The characteristic decomposition feature of sulphides containing a benzyl group is the formation not only of an RS¯ ion, but also the (C7H7) ion. For example, benzyl phenyl sulphide yields PhS¯ (C7H7)¯ ions [22]. On the other hand, (C7H7)¯ on decomposition yields acetylene and (C5H5)¯ ion (Figure 16 (m/e)¯ = 65):
(C7H7)¯ → (C5H5)¯ + CH═CH
Acetylene can readily polymerise, forming a polymer film on the rubbing surface.
Organometallic compounds
There are also examples that show a possible application of the present model to account for some tribological findings connected with Organometallic additives. They encompass metal dialkyldithiophosphates [37] and organometallic compounds having ligands of 8-hydroxychinoline [38]. Recently, it was shown [39] that the tribological effectiveness of complex compounds of Sn(IV) (dicarboxylic acids were used as ligands) in lubrication of steel might be explained in terms of the oxidation reduction reaction
Sn(IV) + Fe(O)  Sn(II) + Fe(II). Sn(II) + Fe(II).
On the other hand, the proposed model can be applied to account for the reduction mechanism of tin from Sn(II) complexes to Sn(O). Evidence was given [39] that tin is present on the steel. This mechanism can be described by reaction of exoelectrons with the Sn(II) complex compound producing the carboxylic anions, iron cations (more specifically, positively charged spots on rubbing surfaces), and Sn(O) atoms. Such a mechanism is supported to some extent by results [37] which showed a possibility of Ni(O) formation from nickel dialkyldithiophosphates.
Addition-type tribopolymerisation
An application of the negative ion-radical model to the polymerisation of vinyl monomers during boundary friction has also been discussed [40]. The formation ofaradical-anion reactive intermediate was assumed. The addition of a monomer to the radical-anion gives a species which contains one radical end and one anion end:
|
|
[CH2═CHX]o— + CH2═CHX → CH2—CHX—CH2—•CHX |
where X is a substituent, e.g. phenyl.
Such species can add monomer from the two ends by different mechanisms. Two radical ends may dimerise, however, leaving a divalent anion to propagate. The formation of radicalanion reactive intermediates is caused by the reaction of low-energy electrons emitted during friction with the monomers
—CH2═CHX + e → [CH2═CHX]o—
Thus, it has to be related to the work function of the rubbing surfaces and to the electron affinity of the monomers involved.
This mechanism has been discussed in terms of tribopolymerisation models as a general mechanism of boundary lubrication [41]. To evaluate the validity of the anionic-radical mechanism, two metal systems were investigated [42, 43], a hard steel ball on a softer steel plate and a hard ball on an aluminium plate. Both metals emit exoelectrons under tribological conditions, but aluminium was found to produce more exoelectrons than steel [13]. The results [42, 43] clearly supported the negative ion-radical concept, showing that addition-type tribopolymerisation can be initiated by exoelectron emission. For example, with aluminium on steel, the addition of 1% styrene to hexadecane reduced the wear volume of the disc by over 65%. In the steel-on-aluminium system, conclusive evidence of polystyrene was found via FTIR microspectrometry on the discs lubricated with styrene-containing solutions.
Most recently, it has been found [44] that lauryl methacrylate, diallyl phthalate and vinyl acetate reduced alumina wear in a pin-on-disc test by 65%, 80% and 57% respectively. However, the antiwear effect of vinyl octadecyl ether under the same friction conditions was marginal. It is believed that the difference can be explained in terms of polymerisation mechanisms. Lauryl methacrylate, diallyl phthalate, and vinyl acetate can polymerise according to anionic or radical mechanisms, whereas vinyl octadecyl ether polymerises only by cationic mechanism. These results are in line with what the negative ion-radical action mechanism predicts: vinyl octadecyl ether, polymerising only by a cationic mechanism, cannot be effective as an antiwear additive for alumina lubrication under the friction conditions described in reference 44.
CONCLUSIONS
The model based upon the ionisation of lubricant components caused by the action of electrons of low energy explains many lubrication phenomena of hydrocarbons, oxygen-containing compounds, and many types of chemicals - including some organometallics - used as antiwear and extreme-pressure additives. It has also been considered as the vinyl type tribopolymerisation mechanism.
References
- Campbell W.E., in Ling, F.F., Klaus, E.E., Fein, R.S. (eds.), Boundary Lubrication, ASME, New York, 1969, ch 6.
- Rowe, C.N., and Murphy, W.R., in Ling, F.F. (ed.), Proc. Tribology Workshop, National Science Foundation, Washington D.C., 1974, p. 327.
- Buckley, DM, Surface Effects in Adhesion Friction, Wear, and Lubrication, Elsevier, Amsterdam, 1981.
- Schey, J.A., Tribology in Metalworking - Friction, Lubrication and Wear, American Society for Metals, Metals Park, Ohio, 1983.
- Heinicke, G., Tribochemistry, Carl Hansen Verlag, Munich, 1984.
- Rosenblum, B., Brauelich, P., and Mimmel, L, "Spontaneous emission of charged particles and photons during tensile deformation of oxide-covered metals under ultrahigh-vacuum conditions', J. Appl. Phys., 48, 5262 (1977).
- Kajdas, C., 'On a negative-ion concept of EP action of organo-sulfur compounds', ASLE Trans., 28, 21 (1985).
- Grohn, H., and Paudert, R., Z. Chem., 3, 89 (1963), according to reference 5.
- Mori, S., Kuriyama, 0., Maki, Y., and Tamai, Y., 'Mechanically initiated reaction of aluminium with alkyl halides', Z. anorg. allg. Chem., 492, 201 (1982).
- Hsu, S.M., and Klaus, E.E., 'Estimation of the molecular junction temperatures in four-ball contacts by chemical reaction rate studies', ASLE Trans., 21, 201 (1978).
- Jaeger, J.C., Proc. Roy. Soc., 56, 203 (1942), according to reference 10.
- Kramer, J., Der metallische Zustand, Vandenhoek and Ruprecht, Goettingen, 1950.
- Connely, M., and Rabinowicz, E., 'Detecting wear and migration of solid film lubricants using simultaneous exoelectron emission', ASLE Trans., 26,139 (1983).
- Perrante, J., 'Exoelectron emission from a clean annealed magnesium single crystal during oxygen adsorption', ASLE Trans., 20, 328 (1977).
- Kobzev, N.I., Exoelectronic Emission, Foreign Publishing House, Moscow, 1962.
- Tamai, Y., 'Role of mechanochemical activity in boundary lubrication', Suh, N.P., and Saka, N. (eds.). Fundamentals of Tribology, MIT Press, Cambridge, MA, 1980, p. 975.
- Grunberg, L., The formation of hydrogen peroxide on fresh metal surfaces', Proc. Phys. Soc., 66B, 153 (1953).
- Smith, H.A., and McGill, P.M., The adsorption of n-nonadecanoic acid on mechanically activated metal surfaces', J. Phys. Chem., 67, 1025 (1957).
- Shaw, M., The chemico-physical role of the cutting fluid', Metal Progr., 42, 85 (1942).
- Goldblatt, I.L, "Model for lubrication behavior of polynuclear aromatics', Ind. Eng. Chem., Prod. Res. Dev., 10, 270 (1971).
- Rosenfeld, L, 'Gas release during wear', Wear, 40, 165 (1976).
- von Ardenne, M., Steinfelder, K., and Tuemmler, R., Elektronenanlagerungs-Massenspektrographie organischer Substanzen, -Springer Verlag, Berlin, 1971.
- Baist, W., 'Neue Erkentnisse der Verschleissforschung durch moderne physikalische Messmethoden', Schmierungstechnik, 12, 329 (1965).
- Thiessen, PA, Meyer, K., and Heinicke, G., Grundlagen der Tribochemie, Akademie Verlag, Berlin, 1966.
- Kajdas, C., and Tuemmler, R., 'Analysis of chemical composition of solid-petroleum hydrocarbons by EA mass spectrography with negative ions', Org. Mass Spectrum, 2,1049 (1969).
- Appeldorn, Y.K., and Tao, F.F., The lubricity characteristics of heavy aromatics', Wear, 12, 117(1968).
- Jahanmir, S., and Fischer, T.E., 'Friction and wear of silicon nitride lubricated by humid air, water, and hexadecane', STLE Trans., 31, 41 (1988).
- Jones, W.R. Jr., and Hady, W.F., 'Effect of humidity and wettability additive on phenyl ether lubrication of steel in air and nitrogen to 350°C', NASA TN D-6055, Washington DC, 1970.
- Morales, W., 'Formation of high molecular weight products from benzene during boundary lubrication', NASATM 86966, Washington DC, 1985.
- Montgomery, R.S., The effect of alcohols and ethers on the wear behaviour of aluminium'. Wear, 8, 466 (1965).
- Hironaka, S., and Sakurai, T., The effect of pentaerythritol partial ester on the wear of aluminium', Wear, 50,105 (1978).
- Stinton, H.C., Spikes, A., and Cameron, A., 'A study of friction polymer formation', ASLE Trans., 25, 355 (1981).
- Mori, S., Suginoya, M., and Tamai, I., "Chemisorption of organic compounds on a clean aluminum surface prepared by cutting under high vacuum', ASLE Trans., 25, 261 (1982).
- Allum, K.G., and Ford, J.F, 'The influence of chemical structure of the load-carrying properties of certain organosulfur compounds', J. Inst. Petrol., 51,145 (1965).
- Forbes, E.S., "The load-carrying action of organic sulfur compounds. A review', Wear,15, 87(1970).
- Allum, K.G., and Forbes, E.S., 'The load-carrying properties of organic sulfur compounds. The influence of chemical structure on antiwear properties of organic disulfides', J. Inst. Petrol, 53,174 (1967).
- Kajdas, C., Tuemmler, R., von Ardenne, H., and Schwarz, W., 'Die Bedeutung der EA-Massenspektroskopie fuer die Interpretation der Wirkung von Metalldialkyldithiophosphaten waehrend der Reibungs-schmierung', Zfl-Mitteilungen, 115,107 (1986).
- Kajdas, C., and Fodor, J., 'Action mechanism of load-carrying performance of a new lubricant additive', Trib. Trans., 31, 476 (1989).
- Ozimina, D., and Kajdas, C., 'Tribological properties and action mechanism of complex compounds of Sn(ll) and Sn(IV) in lubrication of steel", ASLE Trans., 30, 508(1987).
- Furey, M., and Kajdas, C., 'Planned formation of polymeric films on rubbing surfaces to reduce wear', 61st Colloid and Surface Science Symposium, ACS, Ann Arbor, Michigan, 21-4 June 1987.
- Furey, M., and Kajdas, C., 'Models of tribopolymerization as an antiwear mechanism', Proc. Jap. Int. Trib. Conf., Nagoya, 1990, Vol. 2, p. 1089.
- Marin-LaFleche, P., A study of tribopolymerization under fretting contact conditions, MSc. Thesis, Dept. of Mechanical Engineering, Virginia Polytechnic Institute and State University, Blacksburg, VA, May 1990.
- Marin-LaFleche, P., Kajdas, C., Furey, M.J., Ward, T.C., and Hellgeth, J;W., 'A study of tribopolymerisation under fretting contact conditions', Proc. 8th International Colloq. Tribology 2000, Esslingen, Germany, 1992, vol. 1, p. 8.4-1.
- Furey, M.J., Kajdas, C., Kempinski, R., andTripathy, B.S., 'Tribopolymerization and behaviour of oxygen-containing monomers in reduction of ceramic wear', Proc. 6th International Congress on Tribology, EUROTRIB '93, Budapest, 1993.
|















![]() Sn(II) + Fe(II).
Sn(II) + Fe(II).