Influence of Temperature on the Tribochemical Reactions of Hexadecane
Czeslaw Kajdas
Warsaw University of Technology, Plock, Poland
Monika Makowska, and Marian Gradkowski
Institute for Terotechnology, Radom, Poland
Keywords : hexadecane, tribochemistry, thermochemical reactions, mechanochemistry, initiation of chemical reactions, reaction products
Published in: Lubrication Science 15-4, August 2003. (15) 329
Abstract
This paper investigates the tribochemical reactions of n-hexadecane proceeding in a tribosystem lubricated by n-hexadecane at ambient and elevated temperatures. It is hypothesised that, at ambient temperature, reactions are mostly initiated by the mechanical action of the system, and at elevated temperature (200°C) thermochemical reactions should be dominant. An experimental study was performed using a ball-on-disc machine with steel-on-steel mating elements. To analyse wear tracks, Fourier transform infrared microspectrophotometry (FTIRM) and electron spectroscopy for chemical analysis (ESCA/XPS) were used. To investigate chemical changes in the bulk lubricant, gas chromatography coupled with mass spectrometry (GC IMS) was applied. The results provide clear evidence for the hypothesis that two types of oxygenation processes of n-hexadecane under boundary lubrication conditions should be considered. The first, at ambient temperature, is controlled by the mechanical action and the second is clearly controlled by temperature. The analytical techniques applied gave evidence of the formation of some reaction products from hexadecane under boundary lubrication conditions. These products include compounds having Fe-0 bonding (salts and chelates), carbonyl compounds, and iron carbide.INTRODUCTION
Early work on the chemistry of the boundary lubrication of steel by hydrocarbons, including hexadecane, demonstrated that the sliding behaviour of steel lubricated by hydrocarbons under boundary lubrication conditions could be related to chemical reactions at the sliding surfaces, involving metal, hydrocarbon, and oxygen [1]. The results suggested that the reactions occurred at sites where fresh metal surface was exposed by rubbing.
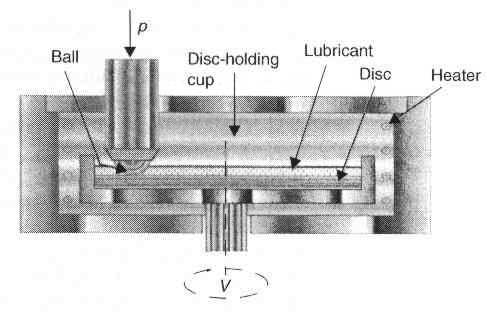 | Figure 1 Ball-on-discT- 11 tester |
EXPERIMENTAL:
Lubricant
A sample of n-hexadecane (>99%; Merck-Schuchardt) was used as the lubricant (model mineral base oil) without any additional purification. Gas chromatographic analysis demonstrated that only one peak assigned to n-hexadecane was present.Tribological tests
To investigate the effects of temperature on tribochemical reactions proceeding under boundary lubrication conditions in a steel-on-steel rubbing system, a T-11 pin-on-disc tester was used. The tester (see Figure 1) was developed by the Institute for Terotechnology in Radom, Poland [7]. This apparatus allows one to run tests at elevated temperatures up to around 300°C. It also continuously records the coefficient of friction and linear displacement due to wear. Elements of the friction pair (balls and discs) were made from 52100 bearing steel, 60 HRC. Before each run, the balls and discs were cleaned ultrasonically in hexane for 15 min.Analytical techniques
FTIRM To investigate the organic layer on the wear tracks an i-series PE Fourier transform infrared microspectrophotometer was used. Reflection spectra were recorded in the wavenumber range 4000-700 cm-1, with a resolution of 4 cm-1 (64 scans at each point). The region of analysis was totally within the disc wear track. The sample spot size was 35 µm x 35 µm. Before each sample analysis, the background spectrum (steel surface outside the wear track) was recorded and automatically subtracted from sample spectra. All sample spectra were corrected by removal of the spurious band originating from carbon dioxide [8], near 2350 cm-1, as well as by smoothing according to Savitsky-Golay's method and multipoint normalising of the baseline. The mathematical processing of the spectra showed no influence on their appearance.ESCA In order to determine the types of chemical bonds present in the products layered on the disc steel surface, the wear track was subjected to ESCA/XPS* analysis. A Phi-5702 multifunctional X-ray photoelectron spectrometer was used with a Mg Ka radiation source and the binding energy of C 1s (284.6 eV) as the reference line. The energy resolution of high resolution spectra was ±0.2 eV. The Fe 2p, C 1s and O 1s profiles were recorded on a 0.8 mm diameter region at a constant pass energy of 93.9 eV. Ion sputtering of the disc surface was carried out with argon ions of 3 keV energy. It was done at a glancing angle of 45° and the depth profile was obtained during 7 min of sputtering.
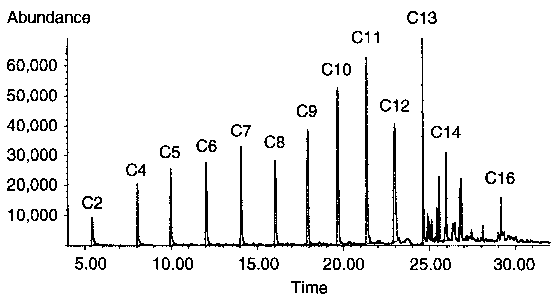 | Figure 2 Result of GC/MS ion 60 (characteristic for monocarboxylic acids) selective analysis of bulk lubricant after friction in steel-steel system (200 °C) |
GC/MS GC/MS was applied to identify the products of bulk lubricant chemical changes proceeding under boundary friction conditions. It was performed using an HP 5890 Series II gas chromatograph coupled with an HP 5972 Series mass spectrometer under the following conditions: injection, 1 µl; column, HP PONA (50 m, diameter 0.2 mm); oven programme, 60-260°C at 8°C/min; pressure programme, 100-200 kPa at 4 kPa/min; flow rate, 0.4 ml/min; carrier gas, He 6.0.
RESULTS AND DISCUSSION
On the basis of the results obtained for elevated temperature and previous results [9] relating to ambient temperature, it is clearly seen that the tribochemistry of n-hexadecane is significantly influenced by elevated temperature, which dramatically changes the oxidation process of this hydrocarbon. The chemically changed bulk lubricant at a temperature of 200°C is composed of various oxygen-containing compounds, especially carboxylic acids (see Figure 2). Other oxygenated products are
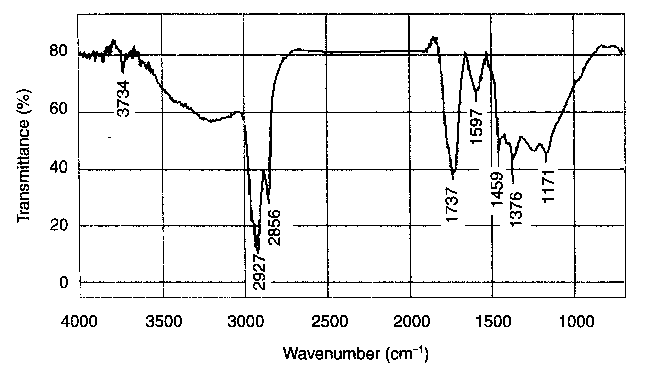 | Figure 3 FTIR spectrum of triboreaction products formed during the friction process carried out at elevated temperature (200 C) |
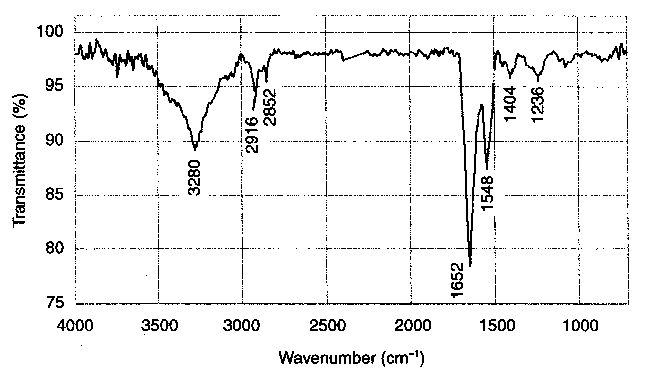 | Figure 4 FTIR spectrum of triboreaction products formed during the friction process carried out at ambient temperature (20°C) |
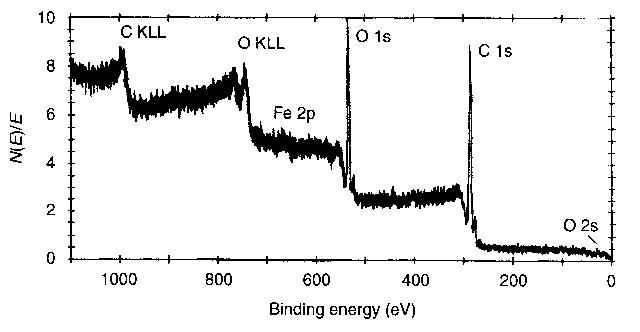 | Figure 5 ESCA survey profile of the steel disc lubricated by n-hexadecane during friction |
alcohols, aldehydes, and ketones. They are typical products of thermooxidative reactions proceeding under high-temperature conditions.
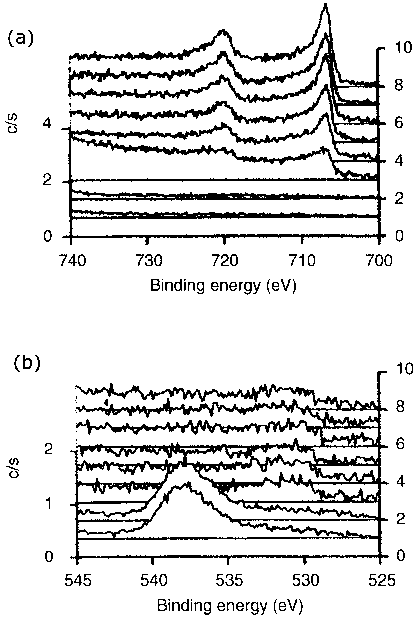 |
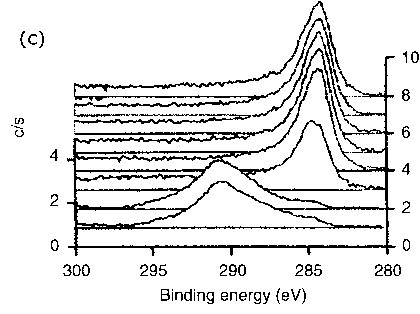 Figure 6 Typical XPS spectra of photoelectrons recorded for the triboreaction product layers formed at elevated temperature (200°C) |
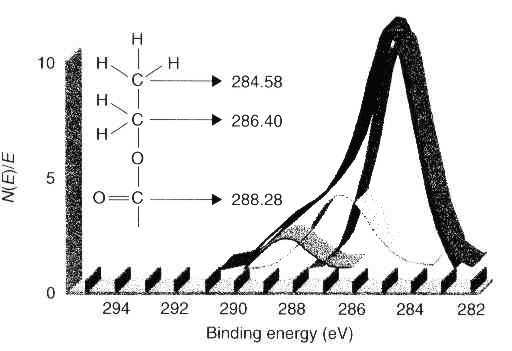 | Figure 7 Result of overlapping peak separation in C 1s photoelectron spectrum |
Table 1 Atomic concentration of elements in the triboreaction product film | ||||||||||||||||||||||||||||||||||||||
| Ion sputtering time (min) | Atomic concentration (%)|
Fe 2p | O 1s | C 1s |
0 | 0 | 28.11 | 71.89 |
1 | 0 | 28.55 | 71.45 |
2 | 20.32 | 1.87 | 77.80 |
3 | 16.17 | 10.39 | 73.44 |
4 | 22.54 | 3.53 | 73.93 |
5 | 23.28 | 2.57 | 74.15 |
6 | 28.27 | 3.56 | 68.17 |
7 | 28.10 | 2.07 | 69.83
| | ||
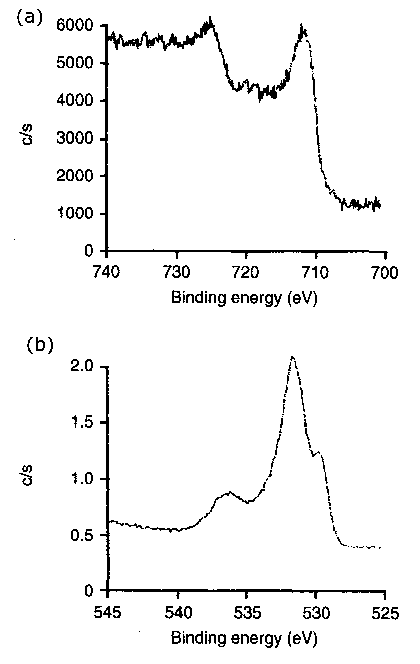 |
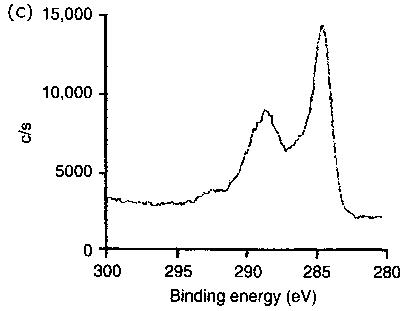 Figure 8 Typical XPS spectra of photoelectrons recorded for the triboreaction product layers formed at ambient temperature (20°C) |
CONCLUSIONS
Using a ball-on-disc tribometer under steel-on-steel boundary lubrication conditions, a study has been made of the tribochemical changes of n-hexadecane initiated by friction and enhanced by elevated temperature. This is part of on-going research into the tribochemistry of lubricant base fluids. The use of FTIRM and ESCA analytical techniques to analyse the surface films and deposits in the contact region showed evidence of the formation of three reaction product types from hexadecane under boundary lubrication conditions. These products include compounds having Fe-O bonding (salts and chelates), carbonyl compounds, and iron carbide.REFERENCES
- Fein, R.S., and Kreuz, K.L, ASLE Trans., 8 (1965) 29-38.
- Hsu, S.M., and Gates, R.S., Proc. Int. Tribology Conference, Yokohama, 1995.
- Nakayama, K., and Hashimoto, H., Wear, 185 (1995) 183-8.
- Jahanmir, S., and Fischer T.E., Trib. Trans., 31 (1988) 32-43.
- Bartelt, G., Lubricants and Lubrication, Proc. 21st Leeds-Lyon Symp. 1994, ed. D. Dowson et al., Elsevier, 1995, pp. 635-57.
- Tripathy, B.S., Furey, M.J., and Kajdas, C., Wear, 181-183 (1995) 138-47.
- Piekoszewski, W., Szczerek, M., and Wulczynski, J., Tribologia, 5-6 (1997) 826-32 (in Polish).
- Nakamoto, K., Infrared and Raman Spectra of Inorganic and Coordination Compounds, John Wiley, New York, 1989.
- Kajdas, C., Makowska, M., and Gradkowski, M., Proc. 9th Nordic Symposium on Tribology, Porvoo, 2000, pp. 594-603.
- Cavdar, B., and Ludema, K.C., Wear, 148 (1991) 329-46.
- Liu, W., Chen, S., Xue, Q., and Zhang, Z., Proc. Symp. on Lubricating Materials and Tribochemistry, Lanzhou, 1998.