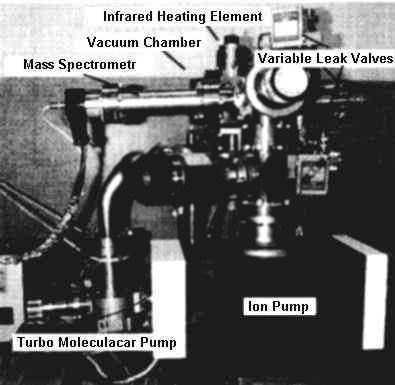
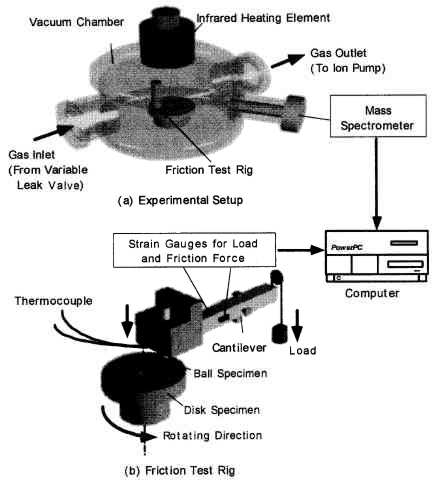
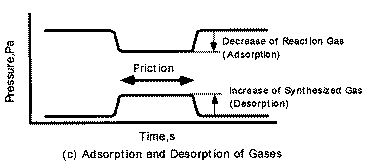
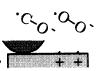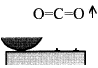| |
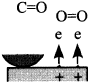
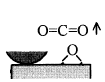
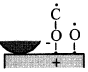
Figure 16 schematically shows the reaction mechanism. Before friction, carbon monoxide and oxygen chemisorb on the surface, which makes domains (Conrad, et al. [20]). If the exposure time is long, chemisorbed atoms are saturated and there are many atoms „waiting for the reaction” on the surface.
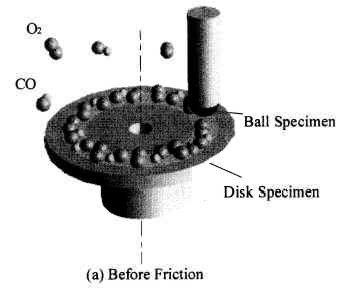 | 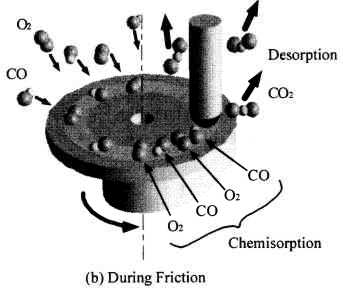 | |
Fig. 16-Schematic of reaction process (not in real scale).
|
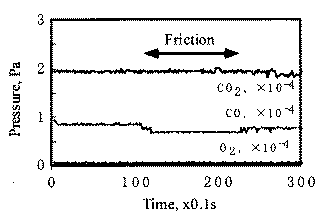
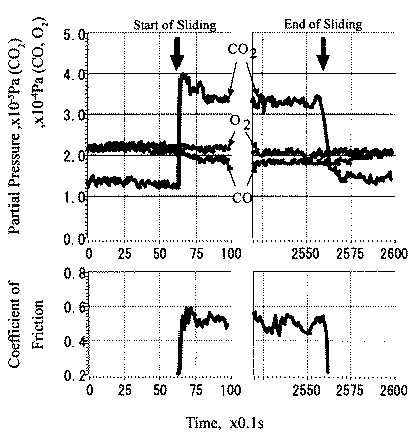
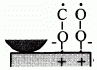
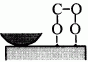
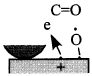
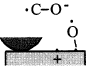
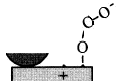
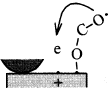
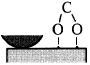
When rubbing starts, exoelectrons are emitted from the surface to promote the NIRAM process. Then carbon dioxide desorbs immediately because it does not chemisorb on the palladium surface. Following the initial period, carbon monoxide and oxygen do not chemisorb on all the worn surface. So after the initial overshoot, the pressure goes down to the equilibrium value during sliding. Stages of the reaction process are schematically shown in Fig. 17.
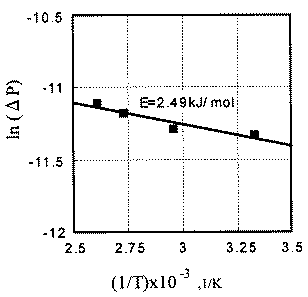
Low activation energy is also explained by the exoelectron emission and NIRAM. The activation energy for the synthesis reaction of carbon dioxide on various palladium surfaces has been studied. It is 121 kJ/mol on (100) surface (Graham, et al. [21]), 105 kJ/mol on (110) surface (Adamson and Gast [22]), and 134 kJ/mol on (111) surface (Gasser [23]). Figure 5 shows, on the other hand, that the apparent activation energy with friction is extremely low. It might be explained as follows: The friction causes the high temperature in a limited duration of time known as flash temperature. The temperature rise associated with friction can equalize the effect of ambient temperature, which results in the low activation energy. However, we need to remember that in high temperature, thermionic electrons are emitted (Vick, et al. [17]). From this context, the high temperature associated with flash temperature is interpreted as a high rate of electron emission. In addition to the thermionic electrons that are emitted during flash temperature, exoelectrons are emitted during the sliding process. These two kinds of electrons equalized the effect of ambient temperature.
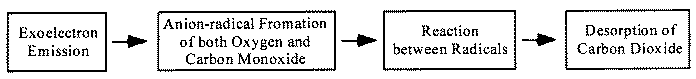 | |
Fig. 17 - Reaction process or the carbon monoxide oxidation by the exoelectron emission and negative-ion-radical formation.
|
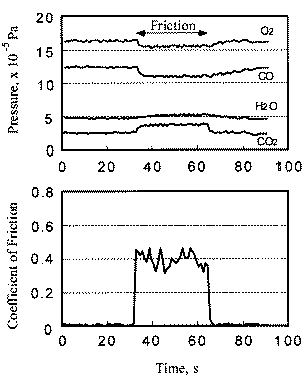
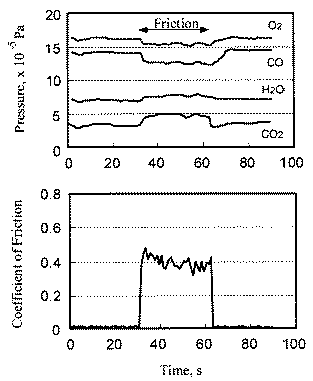
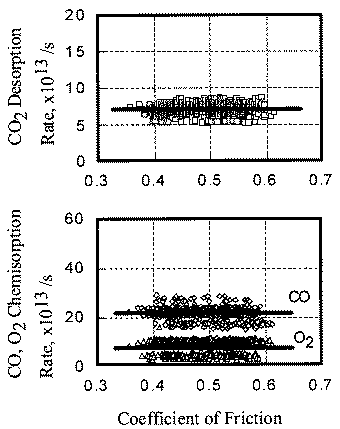
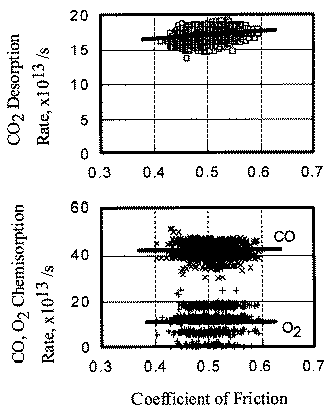
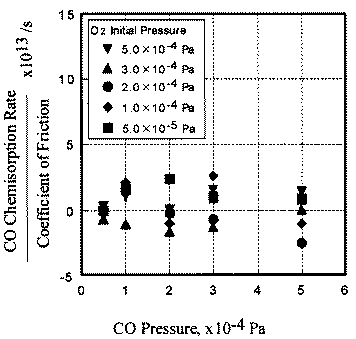
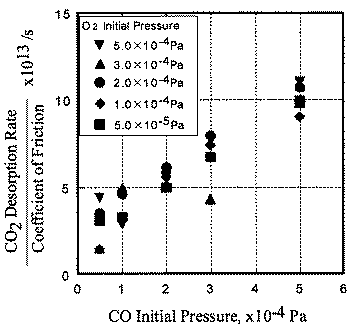
The relationship between coefficient of friction and desorption rate of carbon dioxide is also explained by the exoelectron and NIRAM. It is to be noted that although the variation of the coefficient of friction is very fast, the dcsorption increases or decreases in accordance with the coefficient of friction. This also means that the carbon monoxide and oxygen were ready to react before sliding commenced, and that the exoelectron emission associated with friction triggered the NIRAM process, The friction force originates from the shear fracture at the real area of contact. High friction means larger cuntact area. As a result, exoelectrons are emitted more when the friction is high. Thus the desorption of carbon dioxide correlates positively with the coefficient of friction.
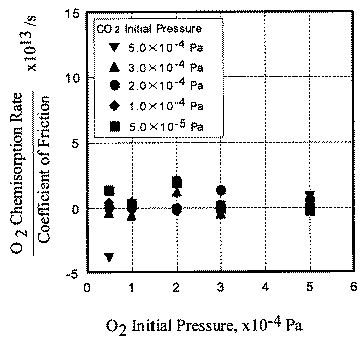
In contrast, the chemisorption occurs not only just after the contact but also during the whole exposure period. This is the reason why chemisorption does not overshoot during the initial period of sliding. No correlation between coefficient of friction and adsorption of carbon monoxide and oxygen clarities the reaction processes, the chemisorption of those molecules is not affected by the exoelectron emission.
CONCLUSIONS
The synthesis reaction of carbon dioxide from carbon monoxide and oxygen is enhanced by friction between aluminum oxide and palladium. The experimental results are summarized as follows:
- The activation energy of this reaction associated with friction was 2,49 kJ/mol.
- The desorption rate of carbon dioxide correlated with the coefficient of friction, whereas the chemisorption rate of carbon monoxide and oxygen had no correlation with it.
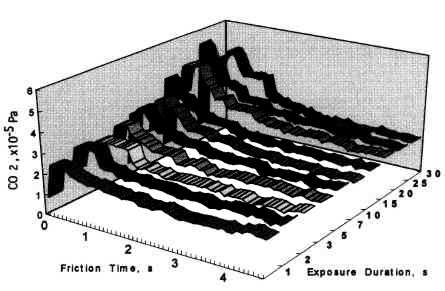
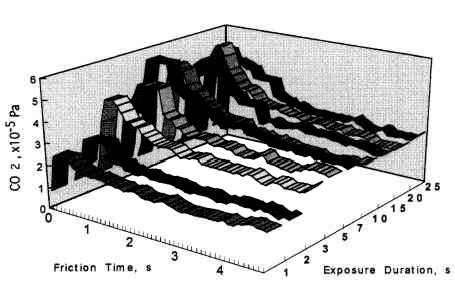
- As the sliding commenced, the dcsorption of carbon dioxide overshot before stabilising at the equilibrium value. The overshoot correlated with the time interval between the friction tests. It was higher with increasing interval time between the friction tests- However, the overshoot did not increase beyond the saturation value.
The experimental findings mentioned above are well explained by exoelectron emission associated with friction and the NIRAM (negative-ion-radical action mechanism) approach. Exoelectron emission is enhanced by the friction of palladium. The exoelectrons attach onto the chemisorbed species to make negative-ion-radicals. Then they react with each other on the surface to be desorbed as carbon dioxide.
| |
REFERENCES
- [1]
- Hiratsuka, K., Kuzuya, M., and Sasada, T. (1990), "Friction Catalysis in the Synthesis Reaction of H2O during Adhesive Wear," Proc. 33rd Japan Congress on Material Research, 33, pp 191-195.
- [2]
- Hiratsuka, K., Kuzuya, M. and Sasada, T. (1991), "Friction Catalysis in the Synthesis Reaction of CO2 during Adhesive Wear," Proc. 34th Japan Congress fin Materials Research, 34, pp 119-123.
- [3]
- Mori, S., Sugjnoya, M. and Tamai, Y. (1982). "Chemisorption of Organic Compounds on a Clean Aluminum Surface Prepared by Cutting under High Vacuum," ASLE Trans., 25, 2, pp 261-266.
- [4]
- Sasada, T., Hiratsuka, K. and Saito, H. (1990), "Adsorption of Surrounding Gas Molecules on Pure Metal Surfaces during Wear Processes" Wear, 135, pp 251-264.
- [5]
- Kajdas, C. ( 1994),''Importance of Anionic Reactive Intermediates for Lubrication Component Reactions with Friction Surfaces," Lubr. Sci. 6, 3, pp 203-228.
- [6]
- Kajdas, C. (1987), "About an Anionic-Radical Concept of the Lubrication Mechanism of Alcohols." Wear, 116, pp 167-180.
- [7]
- Majzner, M. and Kajdas, C. (2003), "Reactions of Carboxylic Acids under Boundary Friction Conditions," Tribologia, 34, 1, pp 63-80.
- [8]
- Kajdas, C. Furey, M. J. and Kempinski, R. (1997). "Tribopolymerizalion: 2. NIRAM Applications to the Antiwear Aclion of Addition-Type Monomers." Proc. 2nd Symposium on Tribochemistry, pp 127-139.
- [9]
- Furey, M. J., Kajdas, C. Kempinski, R. and Tripathy, B. S. (1997), "Action Mechanism of Selected Vinyl Monomers under Boundary Lubrication of an Alumina-on-Alumina System," Lubr. Sci., 10, 1, pp 3-25.
- [10]
- Bhushan, B. and Kajdas, C. (2001), "The Present State of the Art on Degradation Models of Perfluoropolyehers with DLC Coatings in Thin-Film Magnetic Rigid Disks," in Fundamentals of Tribology and Bridging the Gap between the Macro- and Micro/Nanoscales. Kluwer Academic Publishers. Boston, pp 735-746.
- [11]
- Ghasemi, H. M., Furey, M. J. and Kajdas, C. (1993), "Surface Temperatures and Fretting Corrosion of Steel under Conditions of Fretting Contact," Wear 162-164, pp 357-369.
- [12]
- Molina, G. J., Furey, M. J., Ritter, A. L. and Kajdas, C. (2001), Triboemission from Alumina Single Crystal Sapphire, and Aluminum." Wear 349, 3-4, pp 214-219.
- [13]
- Marienfeld, S., Barsotti, S. Leber, E., Schramm, A., Ruf. M. -W., Hotop, H., Rosa, A., Nandi, D., Kumar, S, V. K., Krishnakumar, E. and Ulenberger, E. (2001), "On the Temperature Dependence of SF6— and SF5— Formation in Collisions of Low Energy Electron with SF6 Molecules," XXII International Conference on Photonic, Electronic and Atomic Collisions (XXII ICPEAC) Abstract. MO-050.
- [14]
- Heinicke, G. (1984), Ttibochemistry. Akademie Verlag. Berlin, p 122.
- [15]
- Kajdas, C., Hiratsuka, K. and Hashimoto, M. (2004), "Mechanism of Water Synthesis under Pt/Pt Tribological System in Vacuum." Tribology: Science and Applications, Herman, M. A, ed,, to be published by Polish Academy of Sciences, Warsaw.
- [16]
- Kajdas, C. (2001), "Tribochemistry," in Tribology 2001: Scientific Achievements, Industrial Applications, Future Challenges Franek, F., Bartz, W. J., Pauschitz, A. eds.. Austrian Tribology Society, Vienna, pp 39-46.
- [17]
- Vick, B., Furey, M. and Kajdas, C. (2002), "An Examination of Thermionic Emission Due to Frictionally Generated Temperatures," Tribol. Lett, 13, 2, pp 147-153.
- [18]
- Nakayama, K. and Nevshupa. R. A. (2002). "Plasma Generation in a Gap around a Sliding Contact," J. Phys. D: Appl. Phys. 35, pp 1-4.
- [19]
- Kajdas. C. (2002), "An Attempt to Model the Initiation Process of Tribochemical Reactions by the Flash Temperature Effect," 1st ESF-Tribochemical Nanotribology Workshop in Portovenere, Abstracts, p 19.
- [20]
- Conrad, H., Erll,G. and Kueppers, J. (1978), "Interactions between Oxygen and Carbon Monoxide on a Pd(111) Surface." Surf. Sci., 76, pp 323-342.
- [21]
- Graham, G. W., Logan, A, D. and Shelef, M. (1993). "Oxidation of CO by O2, NO and Mixtures of O2 and NO over Pd(100)," J. Phys. Chem., 97, 21, p5445.
- [22]
- Adamson. A. W. and Gast. A. P. (1997), Physical Chemistry Surfaces, 6th ed, John Wiley. New York. p 736.
- [23]
- Gasser, R. P. H. (1985), An Introduction to Chemisorption and Catalysis by Metals, Clarendon Press, Oxford, p 215.
| |