THE PRESENT STATE OF THE ART ON DEGRADATION MODELS OF PERFLUOROPOLYETHERS WITH DLC COATINGS IN THIN-FILM MAGNETIC RIGID DISKS*
- This work presents and discusses four PFPE degradation models under sliding conditions. The primary goal of the paper is to provide a better understanding of the PFPE lubricant degradation mechanism from the viewpoint of reactive intermediates generation. Emphasis is placed on the PFPE degradation mechanism based on the negative-ion-radical action mechanism (NIRAM) showing that low-energy electrons may play particularly important role in the PFPE degradation process.
2. Degradation Mechanisms of PFPE Lubricants
PFPE lubricant deposited on thin hard carbon coatings under static conditions interacts with the surfaces mostly through physical adsorption forces. The physically adsorbed lubricant film can, however, be also chemically bonded via specific treatment of the deposited film. The known approaches to produce chemically bonded films encompass exposure of the lubricated disk to specific radiation modes, such as- low-energy X-ray (Heideman and Wirth, 1984),
- nitrogen plasma (Homola et al., 1990),
- high-energy ion beam (Lee, 1991),
- low-energy electrons and far ultraviolet (Vurens et al., 1992).
- thermal decomposition process,
- catalytic degradation process,
- electron mediated degradation process, and
- mechanical degradation by shearing process.
2.1. THERMAL DEGRADATION
PFPE as a class of fluid lubricants exhibit excellent thermal and oxidative stability. Notwithstanding the fact that they are thermally stable up to 350°C (Helmick and Jones, 1990), there is clear evidence that their actual thermal stability is adversely affected by the presence of metals. Jones et al. (1983) demonstrated the effects of steel and some titanium alloys and additives on thermal stability of linear perfluoroalkyl ether fluids. Under sliding conditions, particularly at high sliding speed, the temperature on hard carbon coatings can be sufficiently high to initiate PFPE thermal degradation (Bhushan, 1992). For instance, Huu et al. (1997) investigating friction and wear of hard carbon coatings at sliding speeds in the range of 30 to 35 m/s in air, presented evidence that the temperature increase produced by friction in the contact zone was high, about 285°C. Schematic presentation of this most simple free radical PFPE degradation mechanism is depicted in Fig. 1. Presence of oxygen in the environment will lead to the thermal oxidative degradation process in which very reactive peroxide radical can be generated.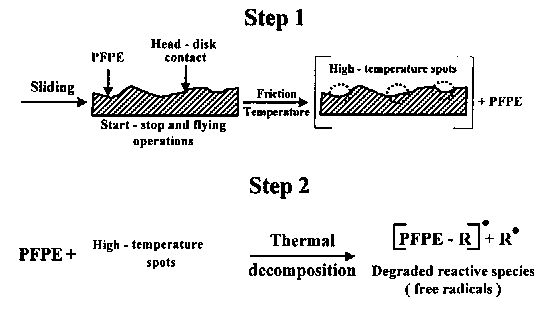
Fig. 1. Thermal degradation process of PFPE lubricants (Temp. >350°C);
R• is a free radical (Kajdas and Bhushan, 1999).2.2. CATALYTIC DEGRADATION
As alpha-alumina exists as the structural element in the construction of head slider for magnetic disk drive, degradation mechanism of PFPEs by a-alumina have been considered (Kasai et al., 1991). Experiments of the degradation process in the presence of AlCl3 have been conducted at high temperatures (Kasai et al., 1991; Kasai, 1999). a-Alumina is a typical Lewis acid, however. AlCl3 is stronger Lewis acid. These studies suggested that the degradation of PFPEs in the presence of Lewis acids (metal oxides or halides) is singularly dominated by the intramolecular disporportionation reactionR1—CF2—O—CF2—O—CF2—R2 → R1—CF3 + FC(O)—R2
where R1 is the left part of Z-DOL molecule from the above shown acetal sector, and R2 is the right portion of Z-DOL molecule.
- This reaction occurs most readily at acetal sector (O—CF2—O), but, given sufficient activation energy and/or in the presence of a stronger Lewis acid, it also occurs at other linkages. It was also found that, when the reaction occurred at the terminal linkage the fluorine transfer was always from the terminal group into the internal sector (Kasai, 1999). Degradation mechanism of Z-type lubricant in the presence of alumina has been characterized as follows (Kasai et al., 1991):
- it is catalyzed by AlF3 formed during the induction period;
- it uniquely involves the acetal moiety (O—CF2—O) of the polymer chains;
- it generates methoxy end-groups (—O—CF3), and
- it results in fragmentation of polymer chains but does not involve an unzipping process.
- Fig. 2 shows schematically the reaction mechanism of the catalyzed degradation mechanism of Z-type lubricant. The reaction sequence is initiated by a bidentate linkage between an acidic aluminum on AlF3 and the two oxygen atoms of an acetal unit, as presented in the upper part of Fig. 2.
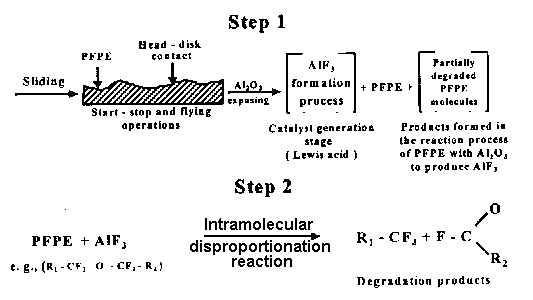
Fig. 2. Schematic presentation of the catalyzed PFPE degradation process (Kajdas and Bhushan, 1999). - The partial positive charge developed at the acetal carbon induces a fluorine atom transfer from the adjacent CF2 unit leading to chain scission with transformation of the acetal unit into a methoxy (—O—CF3) end group, and the adjacent unit into either a fluoroformate F—CO—O—CF2 end group or an acylfluoride F—CO—CF3 end-group from the adjacent unit depending upon whether the adjacent unit was originally a methylene oxide unit or an ethylene oxide unit (Kasai et al., 1991). According to Zhao et al. (2000) catalytic degradation mechanism is not viable because of kinetic of catalytic degradation.
2.3. ELECTRON INDUCED DEGRADATION MECHANISM
Usually, the application of mechanical energy associated with friction releases a great number of physical processes that can be the cause of tribochemical reactions of solids with lubricant molecules. According to Kajdas (1987, 1994), the most important factor governing tribochemical reactions under boundary friction is associated with the action of low-energy electrons with lubricating oil components. This is the basis of the negative-ion-radical action mechanism (NIRAM). In order to find if electrons are emitted at head-disk sliding contacts in magnetic recording media, electron emission phenomena were investigated together with the friction and wear in a frictional system of an alumina ball sliding on amorphous and hydrogenated amorphous carbon films in a dry air atmosphere (Nakayama and Ikeda, 1996). They found that electrons are emitted during wear of both amorphous and hydrogenated amorphous carbon films. The transition from low to high electron emission intensity was caused by the increase in wear of the carbon films.- Bearing in mind that thin films ofperfluoropolyethers can be attached to a variety of substrates, such as amorphous carbon, silica, and gold, by illumination of the substrate with 185-nm UV light (Saperstein and Lin, 1990; Vurens et al., 1992), it is possible to hypothesize that lubricant reactions with head-disk sliding contacts might not be only initiated by temperature and/or catalysis, but also by low-energy electrons emitted from the carbon substrate under the boundary friction conditions. The hypothesis can be strengthened by the fact that the electron bonding is some 3 to 4 orders of magnitude more efficient than the UV bonding process (Vurens et al., 1992).
- The energy of exoelectrons is considered to be very low, of the order of 1 -3 eV (Chaikin, 1967; Dickinson et al., 1997). Grunberg (1958) reviewed the exoelectron emission phenomena. Kajdas (1994) discussed the importance of low-energy electrons for initiation of tribochemical reactions proceeding according to the negative ion-radical action mechanism (NIRAM) approach. The principal thesis of the model is that lubricant molecules, for example alcohols (Kajdas, 1987), form anions which are then chemisorbed on the positively charged areas of rubbing surfaces. Vurens et al. (1992) examined the bonding of the thin PFPE films by low-energy electrons and UV. They provided evidence that the bonding takes place through interaction of the perfluoropolyether molecule, with a low-energy photoelectron emitted by excitation of the substrate by the UV photons, and demonstrated that for a Demnum S200 film on a diamond-like carbon substrate bombarded with low-energy electrons, the most abundant fragment ions comprise the following negative species F¯, C3F5O2¯, and C3F7O¯. Therefore, the electron mediated degradation mechanism of perfluoropolyether lubricants seems to be of particular importance.
- Zhao et al. (2000) conducted head-disk interface experiments by sliding alumina-titanium carbide slider on a rigid disk with an a-C:H overcoat and PFPE film. They used an UV lamp to generate low energy electrons. They reported that the decomposition of lubricant is accelerated in the presence of the low energy electrons. This observation suggests that low-energy electron-induced degradation is a viable mechanism. Schematic presentation of the low-energy electron-induced PFPE degradation process is shown in Fig. 3.
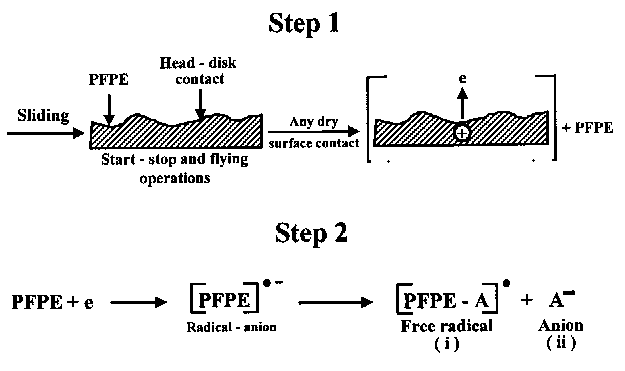
Fig. 3. Schematic showing the electron-induced PFPE degradation process (Kajdas and Bhushan, 1999). 2.4. MECHANICAL DEGRADATION
- Application of an adequate shear stress results in ordering of the PFPE chain and a corresponding decrease in friction (Hirz et al., 1992). The removal of PFPE lubricant molecules form sliding and flying tracks, which comprises the displacement and loss depends on film thickness, chemical structure, and molecular weight. Microscale friction and properties of molecularly thin liquid films most recently were discussed in detail by Bhushan (1999). In flying and sliding, the lubricant removal rate from monolayer films is significantly slower than form multilayer films (Novotny and Baldwinson, 1991). The lubricant loss in sliding can be due to polymer scission (Buchholz and Wilson, 1986). Direct mechanical scission at shear rates of 108 - 1010 S -1 is possible (Novotny and Baldwinson, 1991; Bhushan, 1999; Zhao et al., 1999), however, other factors can contribute to the lubricant degradation as well. Mechanical scission of PFPE bonds leads to the formation of free radicals, similarly as in the thermal degradation process. Fig. 4 provides schematic presentation of the PFPE degradation process caused by high shear stresses.
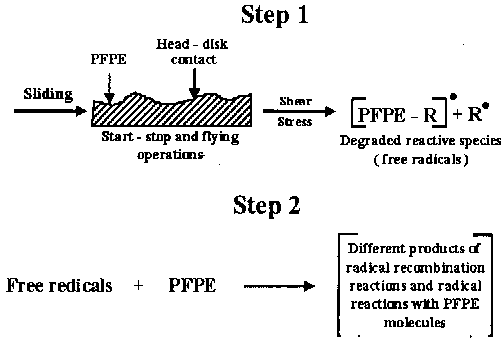
Fig. 4. PFPE degradation process caused by high shear stresses (Kajdas and Bhushan, 1999). 3. Discussion of the Mechanisms
- Under disk drive operation conditions, the head slider slides on the disk surface during start-up and stop operations. Contact start stop (CSS) operations lead to elastic and plastic deformation of asperities and some wear. The lubricant molecules are exposed to the frictional energy which are both physically and chemically adsorbed. The adsorbed and chemically bonded PFPE molecules undergo important changes caused by physical and chemical processes. Physical changes include mechanical displacement of the molecules and their possible desorption and/or evaporation. Chemical changes are much more complex and can proceed according to different mechanisms. The common denominator of all these specific mechanisms is the lubricant degradation process leading to its loss mostly in the form of gaseous species generated at the sliding contacts. For detection of gaseous species generated by sliding in a high vacuum chamber the most widely used measurement technique relates to the quadrupole mass spectrometry. Data concerning the detection of gaseous wear species from lubricated thin film disks have been presented by Bhushan and Ruan (1994), Bhushan and Cheng (1997); Li and Bhushan (1997), Zhao et al. (2000).
- Li and Bhushan (1997) studied the degradation process of Z-DOL and Fomblin AM2001 lubricants on the thin-films disks with an Al2O3-TiC slider under high vacuum. Gaseous products generated from the head/disk interfaces were detected and monitored as a function of sliding distance using a quadrupole mass spectrometer. They found that the gaseous products generated from these two lubricants are quite similar. Decomposition of both lubricants was found to take place from the beginning of sliding, and the evolution rate of these gases decreased with the sliding time. Surface roughness of the disk has an effect on the sliding distance to failure, but little effect on the decomposition of lubricant.
- Using the same set-up, Bhushan and Cheng (1997) investigated three kinds of lubricants with non-polar and polar ends and with and without thermal treatment were applied over a thin-film disk with DLC overcoat. They found that fluorocarbon fragments are generated from lubricants during a period of sliding with a low and stable coefficient of friction, followed by a sharp rise in frictional force and generation of gaseous products of DLC overcoat material. It was concluded that decomposition of PFPE lubricants begins from the onset of sliding, and the DLC overcoat starts to wear simultaneously from the beginning of the sliding for untreated lubricants but was well protected in the case of chemically bonded lubricants. Detailed degradation mechanisms of PFPE lubricants is the most complex and arguable issue.
- Most recent high vacuum test results (Zhao and Bhushan, 1999) demonstrate that the degradation produces of the 1.5 nm thick Z-DOL lubricant film generate CFO, HCF2 and CF3, species during sliding. CF2O fragment has not been detected. Another extensive work (Zhao et al., 2000) also includes Z-DOL lubricant degradation process and clearly shows the importance of both CFO and CF2O species along with HCF2, CF3 and other fragments. The formed Z-DOL degradation fragments are accounted for in terms of the electron induced degradation mechanism. For example, to account for the CF2H and CFCF3 Z-DOL degradation fragments detected in mass spectra under UHV sliding conditions, electron-induced degradation mechanism was proposed (Zhao et al., 2000).
- Interestingly, the electron induced degradation mechanism can also be applied in accounting for experimental results concerning chemical bonding of PFPE lubricant films with DLC under sliding conditions. Most recent research (Zhao et al., 1999) demonstrated how low-energy electrons may attach to the PFPE lubricant molecules and allow the chemical reactions between lubricant molecules and a DLC surface. Fig. 5 depicts the steps through which lubricant Z-DOL molecules are chemically bonded to the DLC surface after sliding.
- Under sliding conditions, low-energy electrons are emitted at the interface (Step 1); these electrons then attach to lubricant molecules forming reactive species (Step 2). The generated negative-ions (—CF2—CH2—O¯) react with the surface and form bonded molecules (Step 3). Hydrogen atoms (free radicals) recombine and produce hydrogen molecules H2, or they can interact with dangling bonds on the surface. Fig. 6 describes the steps through which lubricant Z-15 or Z-25 molecules are chemically bonded to the DLC surface after sliding.
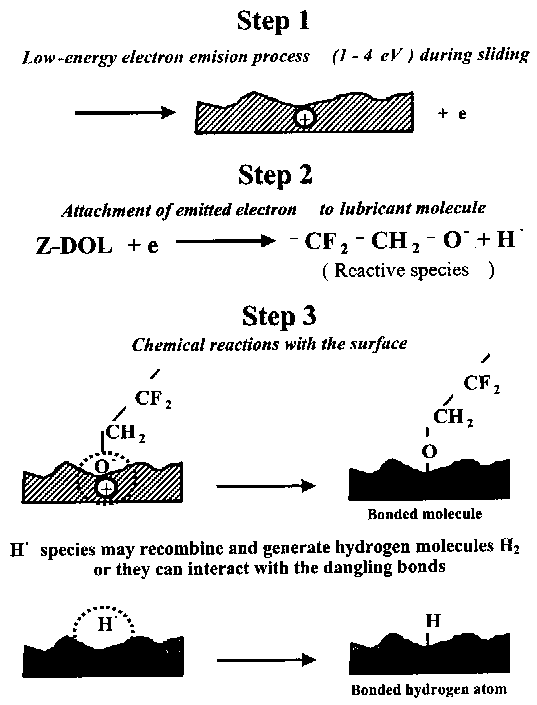
Fig. 5. Interaction of emitted electrons with Z-DOL lubricant molecules and subsequent interactions with the surface (Zhao et al., 1999) - Step 1 is similar to that in Fig. 5. The low-energy electrons then attach to lubricant molecules and form the reactive species (Step 2). The produced negative-ion reactive species allows chemical reaction with the surface and forms bonded molecules; free radical species can react with the surface dangling bonds (Step 3a). Free radicals can also recombine, forming a PFPE molecule, or they can interact with Z-15 lubricant molecules producing a smaller PFPE compound and generating another free radical [Z-15 - F]•. The latter radical can recombine with •CF2 - free radical, generating a higher molecular-weight lubricant compound. More research is needed, however, to better understand all these processes.
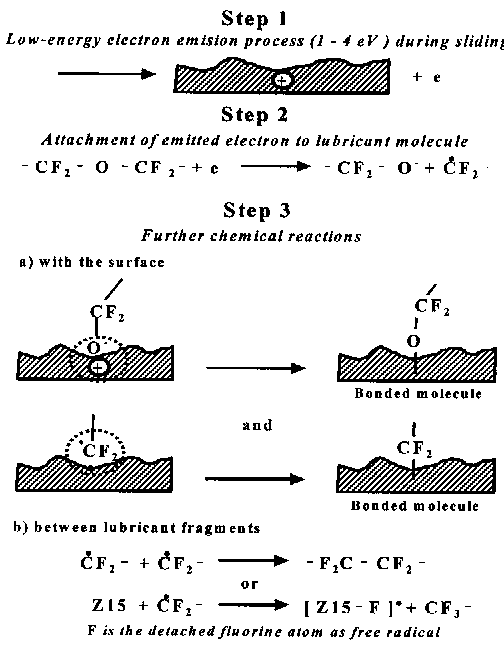
Fig. 6. Interaction of emitted electrons with Z-15 or Z-25 lubricant molecules and subsequent interactions with the surface (Zhao et al., 1999). 4. Conclusion
Based on the reviewed/discussed experimental data it is concluded that anionic intermediates (negative ions and/or negative-ion-radical species) produced by low-energy electrons play an important part in both- the electron mediated degradation process of PFPE lubricants, and
- chemical bonding of PFPE lubricant films with DLC surfaces under sliding conditions.
References
- Bhushan, B. (1992), "Magnetic Head-Media Interface Temperatures. Part 3: Applications to Rigid Disks," ASME J. Tribology, 114, 420-430.
- Bhushan, B. (1996), Tribology and Mechanics of Magnetic Storage Devices, second ed., Springer-Verlag, New York.
- Bhushan, B. (1999), Principles and Applications of Tribology, Wiley, New York. Chapter 11 (Micro/Nanotribology).
- Bhushan, B. and Cheng, Y. (1997), "Wear and Degradation Mechanisms of Magnetic Thin Film Rigid Disks with Different Lubricants Using Mass Spectrometry," J. Appl. Phys. 81, 5390-5392.
- Bhushan, B. and Ruan, J. (1994), "Tribological Performances of Thin Film Amorphous Carbon Overcoats for Magnetic Recording Disks in Various Environments," Surface and Coating Technol. 68/69, 644-650.
- Buchholz, F. L. and Wilson, L. R. (1986) "High Shear Rheology and Shear Degradation of Aqueous Polymer Solutions," ./. Appl. Poly. Sci. 32, 5399-5413.
- Chaikin, S.W. (1967), "On Frictional Polymer," Wear 10, 49-60.
- Dickinson, J. T., Scudiero, L., Yasuda, K., Kirn, M-W. and Langford, S. C. (1997), "Dynamic Tribological Probes: Particle Emission and Transient Electric Measurements," Tribol. Lett. 3, 55-67.
- Grunberg, L. (1958), "A Survey of Exoelectron Emission Phenomena," Brit. J. of Appl. Phys. 9, 85-93.
- Heideman, R. and Wirth, M. (1984), "Transforming the Lubricant on a Magnetic Disk into a Solid Fluorine Compound," IBM Tech. Disclosure Bull. 27, 3199-3205.
- Helmick, L. S. and Jones, W. R. (1990), "Deformation of the Thermal Stability of Perfluoroalkylethers," NASA TM-102493, NASA, Washington, D.C.
- Hirz, S. J., Homola, A. M., Hadziiannou, G. and Frank, C. W. (1992), "Effect of Substrate on Shearing Properties of Ultrathin Polymer Films," Langmuir 8, 328-333.
- Homola, A. M., Lin, L. J. and Saperstein, D. D. (1990), "Process for Bonding Lubricant to a Thin Magnetic Recording Disk," U.S. Patent 4,960,609, October 2.
- Huu, T. Le, Zaidi, H. and Paulmier, D. (1997), "Friction and Wear Properties of Hard Carbon Coatings at High Sliding Speed," Wear 203-204, 442-446.
- Jones, W. R., Paciorek, K. J. L., Ito, T. I. and Kratzer, R. H. (1983), "Thermal Oxidative Degradation Reactions of Linear Perfluoroalkyi Ethers," Ind. Eng. Chem. Prod. Res. Dev. 22, 167-170.
- Kajdas, C. (1987), "About an Anionic-Radical Concept of The Lubrication Mechanism of Alcohols," Wear 116, 167-180.
- Kajdas, C. (1994), "Importance of Ionic Reactive Intermediates For Lubricant Component Reactions With Friction Surfaces," Lubr. Science 6, 203-228.
- Kajdas, C. and Bhushan, B. (1999). "Mechanism of Interaction and Degradation of PFPEs with a DLC Coating in Thin-Film Magnetic Rigid Disks: A Critical Review", J. Info. Storage Proc. Syst. 1, 303-320.
- Kasai, P. H. (1999), "Degradation of Perfluoropolyethers and Role of X-1P Additives in Disk Files," J. Info. Storage Proc. Syst. 1, 22-31.
- Kasai, P. H., Tang.W.T. and Wheeler, P. (1991), "Degradation of Perfluoropolyethers Catalyzed by Alumina Oxide," App. Surf. Sci. 51, 201-211.
- Lee, T.D. (1991), "Enhanced Chemical Bonding Between Lubricants and Magnetic Coatings by High Energy Irradiation and its Application to Tape Durability," J. Vac. Sci. Technol. A9, 1287-1292.
- Li, Y. and Bhushan., B. (1997), "Degradation of Perfluoropolyether Lubricants on Magnetic Recording Disks During Sliding," Proc. Inst. Mech. Engrs. Part J 211, 279-288.
- Nakayama, K. and Ikeda, H. (1996), "Triboemission Characteristics of Electrons During Wear of Amorphous Carbon and Hydrogenated Amorphous Carbon Films in a Dry Air Atmosphere," Wear 198, 71-76.
- Novotny, V. J. and Baldwinson, M. A. (1991), "Lubricant Dynamics in Sliding and Flying," ./. Appl. Phys. 70, 5647-5652.
- Saperstein, D. and Lin, L. (1990), "Improved Surface Adhesion and Coverage of Perfluoropolyether Lubricants Following for-UV Irradation," Langmuir 6, 1522-1524.
- Simonds, J. L. (1995), "Magnetoelectronics Today and Tomorrow," Physics Today, April, 6-32.
- Vurens, G. H., Gudeman, C. S., Lin, L. J. and Foster, J. S. (1992), "Mechanism of Ultraviolet and Electron Bonding of PFPEs," Langmuir 8, 1165-1169.
- Zhao, Z. and Bhushan, B. (1999), "Tribological Performance of PFPE and X-1P Lubricants at Head-Disk Interface - Part I. Experimental Results," Tribol. Lett. 6, 129-139.
- Zhao, Z., Bhushan, B. and Kajdas, C. (1999), "Effect of Thermal Treatment and Sliding on Chemical Bonding of PFPE Lubricant Films with DLC Surfaces," J. Info. Storage Proc. Syst. 1, 259-264.
- Zhao, X., Bhushan, B., and Kajdas, C. (2000), "Lubrication Studies of Head-Disk Interfaces in a Controlled Environment - Part II: Degradation Mechanisms of PFPE Lubricants, " Proc. Inst. Mech. Engrs., Part J (in press).
BHARAT BHUSHAN
Computer Microtribology and Contamination Laboratory, The Ohio State University, 206 W. 18th Avenue, Columbus, Ohio 43210, U.S.A.
CZESLAW KAJDAS
Warsaw University of Technology, Institute of Chemistry in Plock, 17 Lukasiewicza Street, 09-400 Plock and Central Petroleum Laboratory in Warsaw, Poland
* — Published in : B. Bhushan (ed.), Fundamentals of Tribology and Bridging the Gap between the Macro- and Micro/Nanoscales, 735-745. 2001 Kluwer Academic Publishers. Printed in the Netherlands.