Tribological performance of PFPE and X-1P lubricants at head-disk interface. Part II. Mechanisms
Zheming Zhaoa, Bharat Bhushana and Czeslaw Kajdasb
* — Published in : Tribology Letters Vol., 6, (1999) 141-148
1. Introduction
Perfluoropolyether (PFPE) lubricants, such as Fomblin Z-Dol and Demnum SA, are popularly used in rigid disk drives [1]. However, one of the major tribological problems at the head-disk interface is the decomposition of PFPE lubricants, resulting in wear of the carbon overcoat consequently head-disk interface failure. Advanced lubricants for disk drive applications are continually developed for high-density recording, proximity recording and even contact recording. Lubricant X-1P is recently used as an additive to PFPEs in disk drives. Fomblin Z-type and Demnum SA lubricants are a random copolymer of CF2CF2O and CF2O with a linear structure whereas X-1P is a mixture of p-fluorophenoxy- and m-trifluoromethylphenoxy-substituted cyclic phosphazenes, with a spherical structure; it contains four m-trifluoromethylphenoxy and two p-fluoro-phenoxy substituted groups (table 1). It is known that PFPE lubricants are more likely to be degraded at wet environmental condition if alumina is used as a mating material whereas lubricant X-1P is much less degraded. Understanding of the PFPE lubricant decomposition and X-1P's role is required to meet the need of the continuously increased areal density in magnetic recording.
 | etc |
Thus, the interaction between polar lubricant atoms (H and O, as in Z-Dol) and/or surface hydroxyl groups can be very significant. In Z-15, even though the lone pairs of electrons in the fluorine atom are unshielded and thus exhibit electronegativity, the —CF3 end is symmetric so its polarity is low and negligible as compared to the polarity of the —OH end. Also, X-1P is a non-polar lubricant.
|
Table 1. Chemical structure and selected properties of perfluoropolyether lubricant Z-15, Z-Dol and SA, and lubricant X-1P. | |||||
| Lubricant | Formula | End Groups | Molecular Weight (Daltons) | Density (x10³ kg/m³ at 20°C) | Kinematic viscosity (mm²/s) |
| Z-15 | CF3—O—(CF2—CF2—O)m—(CF2—O)n—CF3 | —CF3 | 9100 | 1.84 | 150 @ 20°C |
| Z-DOLa | HO—CH2—CF2—O—(CF2—CF2—O)m—(CF2—O)n—CF2—CH2—OH (m/n ~ (2/3) | —OH | 2000 | 1.81 | 80 at 20°C 34 at 40°C |
| SAa | F—(CF2—CF2—CF2—O)m—CF2—CF2—CH2—OH | —OH | 3600 | 1.87 | 75 @ 40°C |
| X-1P | 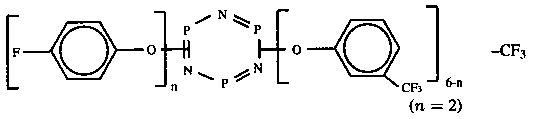 | —CF3 | 1000 | 1.48 | 1826 @ 20°C 205 @ 40°C |
|
| |||||
2. Importance of low-energy electrons in the PFPE lubricant degradation process
2.1. A survey of degradation mechanisms
There are four mechanisms considered to decompose the PFPE molecules: (1) thermal decomposition reactions by frictional heating; (2) catalytic reactions by materials of the head sliders or disks; (3) the scission of the PFPE molecules by mechanical shearing, and (4) triboelectrical reactions by electrons emitted from the wearing solid surfaces. Thermal decomposition temperature of the PFPE lubricants has been reported to be above 350 °C [6] which can be reached by the transient temperature on microsecond scale [1,7]. The decomposition can occur by oxidation and chemical breakdown at high interface temperatures. Kasai et al. [8] and Kasai [9] reported that Lewis acid sites present on Al2O3 are catalytic and cause PFPE lubricant molecules decomposition. Lewis acid is defined as a substance that can accept a pair of electrons. Their experiments had been conducted with ground-up powders of Al2O3 in the presence of lubricant at high temperatures ranging from 200 to 300 °C for a duration of 1-48 h. Kinetics of these experiments is very different from that in the drive operation. Novotny et al. [10] anticipated that PFPE lubricants are decomposed by the scission of the polymer chains, driven by several processes - high shear rates, high interfacial temperature and tribocharging. Perettie et al. [11] conjectured that there was no decomposition of X-1P in contact with the Al2O3—TiC surface although no data were presented to support their evidence. Vurens et al. [12] reported the evidence that decomposition of PFPEs during wear was an electron-mediated process. In their analytical investigation into the degraded lubricant X-1P, Keller and Saba [13] reported that oligomerization to be the dominant mode of degradation, resulting in most of the observed viscosity increases. Their GC/MS, FT-IR, and P NMR data confirmed the existence of a cyclotetramer structure in the degraded fluid.2.2. Tribo-emission
The tribo-emission may be a significant factor in tribochemical reactions in the contact zone (operating under lightly loaded conditions) in addition to temperature rise and the catalytic action of the sliding surface. It is known that a wide variety of materials after mechanical treatment become electron emitters. The term "exoelectron emission" originates from investigation of the emission in freshly formed metals which were accounted for as a consequence of exothermal transformation process of the surface [14]. As reported in the literature [15-19], exoelectron emission occurs when a material's surface is disturbed by plastic deformation, abrasion, fatigue cracking or phase transformation. The electron emission from a freshly formed surface reaches a maximum almost immediately then decays with time [15,20]. Electron emission has been observed from both metals and nonmetals. The energy of exoelectrons is considered to be very low, of the order of 2-3 eV [21]. At present, the term exoelectron emission is used as an inclusive term for emission phenomena which proceed unsteadily, with changed work function, and after excitation by non-thermal energy. Recently, Tagawa et al. [22] investigated the tribo- and photo-stimulated exoelectron emission from reactor graphite disk against pin (material SUS304). They found that the variation of tribo-stimulated exoelectron emission from graphite corresponded well with the variation of the friction coefficient. Figure 1 shows the changes in the emission intensity from a graphite disk and the friction coefficient over 0-4 revolutions of sliding in a pin-on-disk tester.
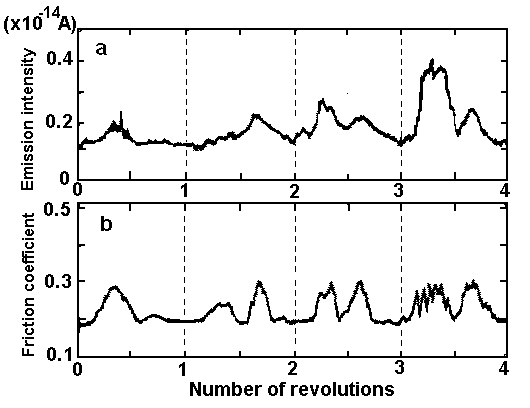 | Figure 1. Variations of (a) tribo-stimulated exoelectron emission and (b) friction coefficient, obtained from reactor graphite sliding against SUS304 pin in a pin-on-disk test [22]. |
 |
|
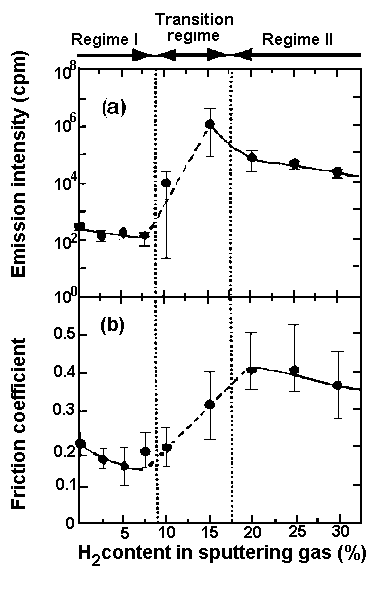
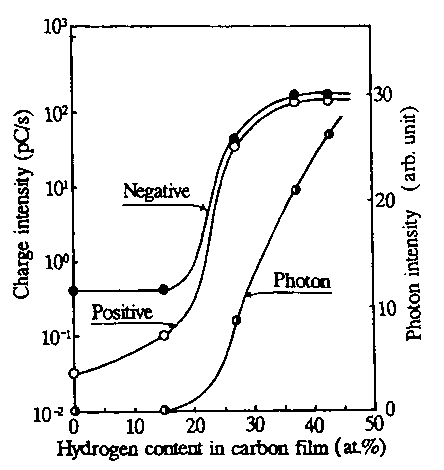 | Figure 3. Relation between charge intensity accumulated for 1 s and hydrogen content in carbon film sliding against diamond stylus in a pin on-disk test [24]. |
With intermediate level hydrogen gas content, transitions of friction, wear intensity and electron emissions occurred from low to high. At high hydrogen gas content, high friction and high wear occurred to produce high electron emissions. Figure 2 shows the dependence of the triboemission intensity of electrons in counts/min and the friction coefficient on hydrogen gas content in the sputtering gas in the sliding test of alumina sliding on amorphous-hydrogenated carbon (a:C) and a:C-Hx; films. Most recently, Nakayama et al. [24] investigated tribo-emission of negatively charged particles and positively charged particles, tribocharging and the friction coefficient in a frictional system with diamond sliding on hydrogenated carbon films in ambient air. They found that in the region of low hydrogen content, at and below 15 at%, both negatively and positively charged particles were emitted, but no photon emission was observed. In the region with hydrogen contents between 15 at% and 37 at%, emission intensity of the charged particles increased sharply, accompanied by tribo-photons. Figure 3 clearly depicts this situation and shows that with a further increase in hydrogen content, the emission intensity of the negatively and positively charged particles and photons increased. Interestingly, the friction coefficient also increased from a low to a high value in the regime of hydrogen content between 15 at% and 27 at%, so that the regime of charge transition and friction transition do not correspond [24]. The attachment of the emitted electrons degrades lubricant molecules through C—O bonding scission in Z-Dol.
2.3. Negative-ion-radical action mechanisms
3. Interaction details on the head-disk interface
3.1. Role of adsorbed water film
|
O═C—OR |
• Danglig bonds; — Covalent bond, ↔ Hydrogen bond interaction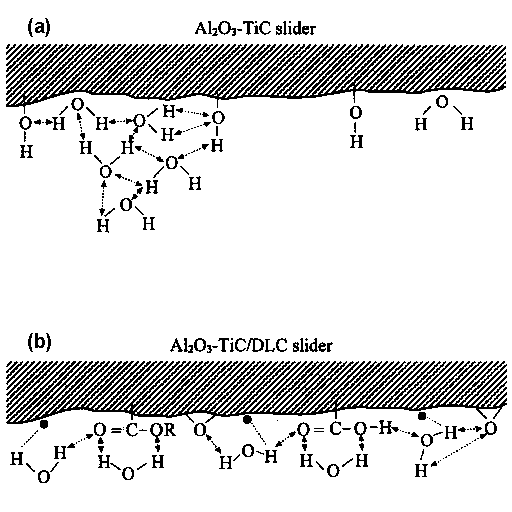 |
|
One of conceivable interaction between water molecules and the DLC atom can proceed through the water hydrogen atom and the surface dangling bond. Dangling bonds are free radicals present in DLC overcoats. A free radical is an atom or group of atoms having one or more unpaired electrons. It is symbolized with a single dot representing the unpaired electron, for example: F•; HO•; CF3•; CF3—O•; CF3—CF2• etc. They have no positive or negative charge, however, they are very reactive. A free radical is highly reactive intermediate because of its unpaired electron.
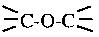 group (number of oxygen atoms per CF2 molecules) than Demnum SA. It is expected that Z-Dol would be more favorable to water molecules. The test results of static friction, kinetic friction and durability have confirmed that lubricant Demnum SA is relatively insensitive to humid environment as compared to that of lubricant Z-Dol [3]. Lubricant X-1P involves no hydrophilic end groups. There is no interaction between its end groups and water molecules. The structure of X-1P makes no hydrogen bonding interactions as existed in PFPE lubricants available at the interface.
group (number of oxygen atoms per CF2 molecules) than Demnum SA. It is expected that Z-Dol would be more favorable to water molecules. The test results of static friction, kinetic friction and durability have confirmed that lubricant Demnum SA is relatively insensitive to humid environment as compared to that of lubricant Z-Dol [3]. Lubricant X-1P involves no hydrophilic end groups. There is no interaction between its end groups and water molecules. The structure of X-1P makes no hydrogen bonding interactions as existed in PFPE lubricants available at the interface.
|
Table 2. Hydrophile-lipophile balance (HLB) number for selected groups. | |
| Group | HLB number |
| Hydrophilic | |
| —COOR | 2.4 |
| —COOH | 2.1 |
| —OH | 1.9 |
| —O— | 1.3 |
| Hydrophobic | |
| —CH2— | -0.475 |
| —CH3 | -0.475 |
| ═CH— | -0.475 |
| —CF2— | -0.87 |
| —CF3 | -0.87 |
| Miscellaneous | |
| —(CH2CH2O)— | 0.33 |
| —(CH2CH2CH2O)— | -0.15 |
3.2. Tribo-emission at the head-disk interface
3.3. Lubricant X-1P and Z-Dol on DLC overcoat
3.4. PFPE lubricant C—O bond scission
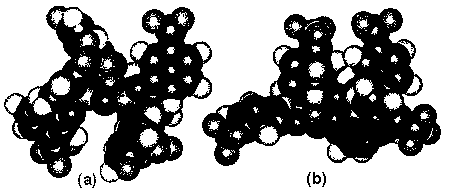 | Figure 5. Structure of (a) planar phosphazene ring and (b) puckered phosphazene ring of X-1P by the molecular mechanics method [37]. |
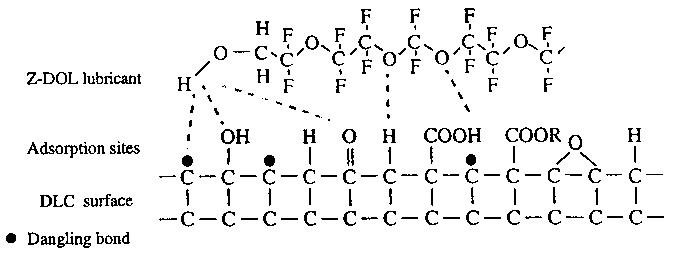 | Figure 6. Schematic for the Z-DOL molecules adsorbed on the DLC surface at 20 °C. |
|
Table 3. Primary bond energies of various bonds in PFPE lubricants. | |
| Bond | Dissociation energy (kJ/mol) |
| C—F | 473 |
| O—H | 464 |
| C—H | 414 |
| C—O | 360 |
| C—C | 347 |
4. Proposed mechanism of PFPE degradation and experimental evidence
- The adsorption process of water molecules is controlled by the surface property. Alumina surface, unless highly dried, contains hydroxyl groups. DLC overcoats include a variety of functional groups.
- Molecules of PFPE lubricants such as Z-Dol are characteristic of partial positive charge on hydrogen and partial negative charge on oxygen.
- Hydrogen bonding interactions at sliding surfaces result in higher friction. The high friction at the interface depletes lubricant film at some sites where solid-solid asperity contact will occur.
- Low energy (1-4 eV) electrons are thus emitted from the sliding surfaces where solid-solid asperity contacts occur. Emitted electrons interact with lubricant molecules.
- Lubricant C—O bond scission is induced through the interaction of low-energy electrons with PFPE lubricant molecules. Lubricant molecules are thus decomposed.
|
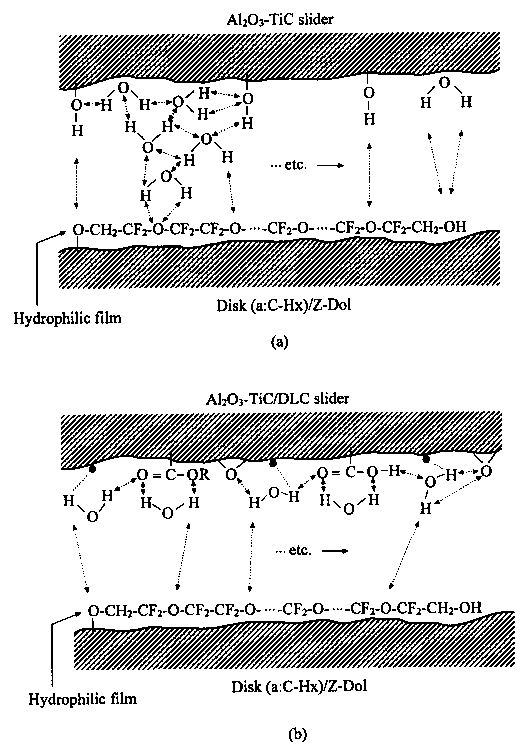 |
|
It is the stronger hydrogen bonding interaction which causes high friction followed by depletion of lubricant film at some sites on the lubricated disk surface. The solid-solid asperity contact at the sliding interface emits the low voltage electrons. The attachment of the emitted electrons degrades lubricant molecules through C—O bonding scission in Z-Dol. Consequently, more solid-solid asperity contact forms followed by more electrons being emitted on the sliding surface. Such a cycle continues until the lubricant film is completely degraded. In the case of DLC coated Al2O3-TiC slider, the carbon coating on the slider surface confines the water molecules and minimizes strong interactions of hydrogen bonding at the interface. The lack of hydrogen bonding interaction keeps friction low and avoids more electrons being emitted from solid-solid contact. Lubricant degradation is postponed.
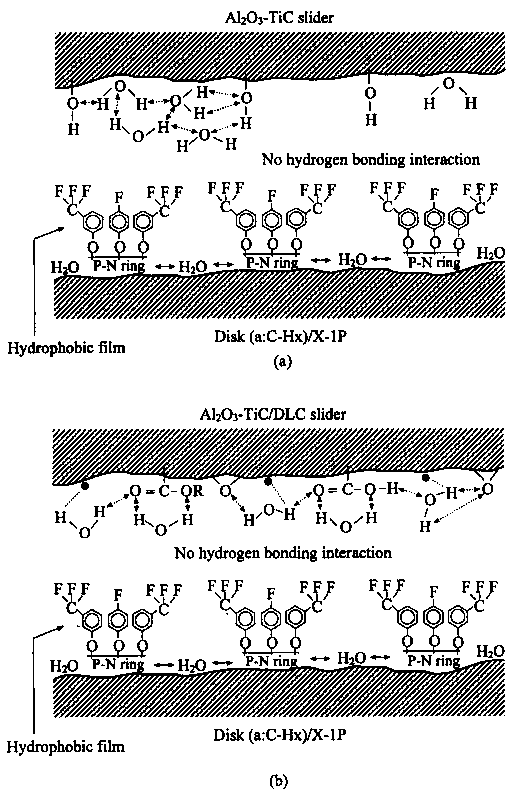 |
|
Some water molecules might be confined within
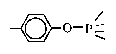
|
groups which can form hydrogen bonding. In the case of the disk surface with some sites not covered by X-1P molecules, water molecules will be adsorbed on the a:C—Hx surface sites and therefore they will be far away from the adsorbed water molecules on the surface of Al2O3-TiC slider. Without the hydrogen bonding interaction, the coefficient of friction remains low. On the other hand, compared to Z-Dol, X-1P's higher viscosity and its spherical molecule structure will provide stronger lubricant film in preventing directly solid-solid asperity contact at the interface. This is confirmed by Yang and Chung [39] that in their pin-on-disk test, the sliding interface provides much higher contact resistance in the case of using lubricant X-1P. Therefore, none of hydrogen bonding interactions results in low friction at the interface; lubricant film will not be depleted so the number of solid-solid asperity contact sites do not increase; the density of low-energy electrons emitted at the sliding interface will not increase. It is the reason why lubricant X-1P is not degraded in drag, CSS and ball-on-flat sliding tests.
5. Conclusions
Acknowledgement
Financial support for this study was provided by the industrial membership of the Computer Microtribology and Contamination Laboratory (CMCL).References
- [1]
- B. Bhushan, Tribology and Mechanics of Magnetic Storage Devices, 2nd Ed. (Springer, New York, 1996).
- [1]
- Z. Zhao and B. Bhushan, Wear 202 (1996) 50.
- [2]
- B. Bhushan and Z. Zhao, IEEE Trans. Magn. 33 (1997) 918.
- [3]
- B. Bhushan and Z. Zhao, J. Info. Storage Proc. Syst. 1 (1999) 1.
- [4]
- Z. Zhao and B. Bhushan, Tribol. Lett. 6 (1999) 129.
- [5]
- L.S. Helmick and W.R. Jones, TM-102493, NASA, 1990.
- [6]
- B. Bhushan, ASME J. Tribol. 114 (1992) 420.
- [7]
- P.H. Kasai, W.T. Tang and P. Wheeler, Appl. Surf. Sci. 51 (1991) 201,
- [8]
- P.H. Kasai, J. Info. Storage Proc. Syst. 1 (1999) in press.
- [9]
- V.J. Novotny, T.E. Karis and N.W. Johnson, ASME J. Tribol. 114 (1992) 61.
- [10]
- D.J. Perettie, W.D. Johnson, T.A. Morgen, K.K. Kar, G.E. Potter, B.M. DeKoven and C.L. Putzig, Adv. Info. Storage Syst. 7 (1996) 157.
- [11]
- G.H. Vurens, R. Zehringer and D. Saperstein, in: Surface Science Investigations in Tribology, Vol. 485, eds. Y.W. Chung, A.M. Homola and G.B. Street (American Chemical Society, Washington, DC, 1992) ch. 9, pp. 169-180.
- [12]
- M.A. Keller and C.S. Saba, Analytical Chemistry 68 (1996) 3489.
- [13]
- J. Kramer, Der Metallische Zustand (Vandenhoek and Ruprecht, Goettingen, Germany, 1950).
- [14]
- L. Grunberg, Brit. J. Appl. Phys. 9 (1958) 85.
- [15]
- F. Brotzen, Phys. Stat. Sol. 22 (1967) 9.
- [16]
- J. Moore and S. Tsang, in: STP 515 (ASTM, Philadelphia, PA, 1972) pp. 113-117.
- [17]
- W. Baxter, Vacuum 22 (1974) 571.
- [18]
- P.A. March and E. Rabinowicz, ASLE Trans. 20 (1977) 315.
- [19]
- C. Veerman, Materials Sci. and Eng. 4 (1969) 329.
- [20]
- S.W. Chaikin, Wear 10 (1967) 49.
- [21]
- M. Tagawa, M. Mori, N. Ohmae, M. Umeno and S. Takenobu, Tribol. International 26 (1993) 57.
- [22]
- K. Nakayama, K. Yamanaka, H. Ikeda and T. Sato, Tribol. Trans. 40 (1997) 507.
- [23]
- K. Nakayama, B. Bou-said and H. Ikeda, ASME J. Tribol. 119 (1997) 764.
- [24]
- C. Kajdas, Wear 116 (1987) 167.
- [25]
- C. Kajdas, Lubrication Science 6 (1994) 203.
- [26]
- G.H. Vurens, C.S. Gudeman, L.J. Lin and J.S. Foster, Langmuir 8 (1992) 1165.
- [27]
- J.B. Peri, J. Phys. Chem. 69 (1965) 220.
- [28]
- J.B. Peri, J. Phys. Chem. 69 (1965) 211.
- [29]
- E.B. Cornelius, T.H. Milliken, G.A. Mills and A.G. Oblad, J. Phys. Chem. 59 (1955) 809.
- [31]
- J.D. Carruthers, D.A. Payne, K.S.W. Sing and L.J. Stryker, J. Colloid and Interface Sci. 36 (1971) 205.
- [32]
- R.R. Bailey and J.P. Wightman, J. Colloid and Interface Sci. 70 (1979) 112.
- [33]
- M. Yanagisawa, in: Tribology and Mechanics of Magnetic Storage Systems. SP-36, Vol. 9 (STLE, Park Ridge, IL, 1994) pp. 25-32.
- [34]
- J.T. Davies and E.K. Rideal, Interfacial Phenomena, 2nd Ed. (Academic Press, London, 1963).
- [35]
- D. Myers, Surfaces, Interfaces and Colloids: Principles and Applications (VCH Publishers, New York, 1991).
- [36]
- H.R. Allcock, Phosphorus-Nitrogen Compounds: Cyclic, Linear, and High Polymeric Systems (Academic Press, New York, 1972).
- [37]
- N.L. Allinger, Adv. in Phys. Org, Chem. 13 (1976) 1.
- [38]
- S. Matsunuma, T. Miura and H. Kataoka, Tribol. Trans. 39 (1996) 380.
- [39]
- Z. Yang and Y.W. Chung, Tribol. Trans. 39 (1996) 974.