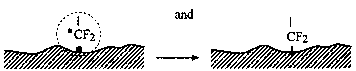Effects of Thermal Treatment and Sliding on Chemical Bonding of PFPE Lubricant Films with DLC Surfaces
Zheming Zhao,¹ Bharat Bhushan,¹ and Czeslaw Kajdas²
* — Published in : J. Info. Storage Proc. Syst, Vol., 1, 259-264, 1999
Abstract
The bonding characteristics of polar perfluoropoly(ether) (PFPE) lubricant Z-Dol and nonpolar PFPE lubricants Z-15 and Z-25 with diamondlike carbon (DLC) surfaces were studied. For lubricant Z-Dol, either prebaking the disk before lubrication or a paper pad's being slid on the lubricated disk was found to enhance the bonding capability of lubricant film on the surface of the magnetic disk with DLC coating. For nonpolar lubricants Z-15 and Z-25, a paper pad's sliding on the lubricated disk resulted in some bonding of lubricant molecules on the DLC disk surface. Experimental results were repeatable. The mechanisms of pre sliding responsible for lubricant bonding are discussed, based on the concept of the hydrogen bond interaction and low-energy electron emission, respectively.1. INTRODUCTION
The liquid lubricants commonly used for thin-film disks are perfluoropoly(ether) (PFPE) lubricants available with or without functional end groups (Bhushan, 1996). Lubricants with —OH end groups are polar lubricants that can chemically bond to the lubricated surface. Attachment of the liquid lubricant molecules has to be enhanced to the overcoat, which, for most cases is sputtered diamondlike carbon (DLC). Surface cleanliness and chemical activity of the overcoat affect the degree of bonding.2. Experimental
The chemical molecular structures of lubricant used in the test are shown in Table 1. For thermal treatment,
 |
Fig. 1. Bonded lubricant film thickness as a function of
|
unlubricated polished disks (type B) with an rms surface roughness of 0.5 nm were used. In each test, two disk coupons were taken from the same disk. One of them was prebaked at 150 °C for 2 h. The disk coupons were lubricated with Z-Dol. Lubricated disks were postbaked at 150 °C at various times. The disks were then washed with solvent FC-72. The bonded fraction of lubricant was measured by using ellipsometry (Zhao and Bhushan, 1996).
3. RESULTS AND DISCUSSION
3.1. Effect of Prebake
Table 2 shows the thickness of the bonded film of lubricant Z-Dol with or without prebaking at 150 °C. The prebaked disks produce a thicker chemically bonded lubricant film, by a factor of 2, compared to that of unbaked disks. For a same postbaking time of 30 min, an increase in the applied lubricant film thickness from 4.7 nm to 12 nm increases the bonded layer thickness from 0.8 nm to 1.0 nm. For the same applied film thickness of 12 nm, the bonded fraction increases from 1.0 nm to 1.2 nm with an increase in the postbaking time from 30 min to 60 min. For disks that were not prebaked, the bonded layer of lubricant film increases from 0.4 nm to 0.5 nm at the same baking time of 30 min with an increase of the applied lubricant film thickness from 4.7 nm to 12 nm. The bonded layer increases a little, from 0.5 nm to 0.6 nm, when the baking time increases from 30 min to 60 min at the applied lubricant film thickness of 12 nm.3.2. Effect of Sliding
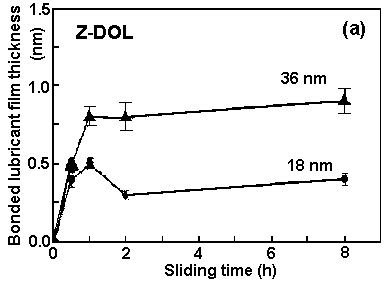
The disks used for this part of the study were neither prebaked nor postbaked. Figure 2 shows the bonded lubricant film thickness as a function of sliding time. For Z-Dol, the bonded layer of lubricant film is higher after sliding in the case of a higher applied film thickness, that is, 36 nm versus 18 nm. The bonded fraction of the film remains at a low level and does not change significantly with the increase in sliding time for an applied film of 18 nm. For a 36-nm applied film thickness, the bonded fraction of the film increases significantly after the sliding time is increased from 0.5 h to 1 h. This indicates that if too thin a lubricant film is applied, it might be easily depleted. The consequence of this is reflected in the low bonded fraction. If the applied film is thick enough, then the increased sliding time enhances the bonding of lubricant to the surface.| TABLE 1. Chemical Structure and Selected Properties of PFPE Lubricants | |||||
| Lubricant | Formula | End Groups | Molecular Weight (Daltons) | Density (x10³ kg/m³ at 20°C) | Kinematic viscosity (mm²/s) |
| Z-25 | CF3—O—(CF2—CF2—O)m—(CF2—O)n—CF3 | —CF3 | 1280 | 1.85 | 250 @ 20°C |
| Z-15 | CF3—O—(CF2—CF2—O)m—(CF2—O)n—CF3 | —CF3 | 9100 | 1.84 | 150 @ 20°C |
| Z-DOL¹ | HO—CH2—CF2—O—(CF2—CF2—O)m—(CF2—O)n—CF2—CH2—OH ; (m/n ~ (2/3) | —OH | 2000 | 1.81 | 80 @ 20°C 34 @ 40°C |
|
| |||||
| TABLE 2. Effect of Prebaking at 150 °C for 2 h on the Bonded Film of Lubricant Z-DOL¹ | |||||
| Lubricant | Postbaking Time (min) | Disk B1 | Disk B2 | ||
| Prebaking | |||||
| Yes | No | ||||
| Applied | 45 | 3.4 nm | 3.4 nm | ||
| Bonded | 45 | 1.0 ±0.08 nm | 0.4 ±0.04 nm | ||
| Applied | 30 | 4.7 nm | 4.7 nm | ||
| Bonded | 30 | 0.8 ±0.06 nm | 0.4 ±0.05 nm | ||
| Applied | 30 | 12 nm | 12 nm | ||
| Bonded | 30 | 1.0±0.09 nm | 0.5 ±0.05 nm | ||
| Applied | 60 | 12 nm | 12 nm | ||
| Bonded | 60 | 1.2±0.09 nm | 0.6 ±0.04 nm | ||
|
| |||||
3.3. Mechanisms
3.3.1. Effect of Prebake
 |
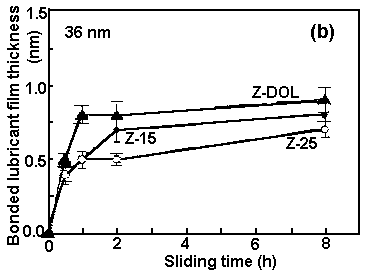 |
| Fig. 2. Effect of sliding time on bonded lubricant film thickness for (a) Z-Dol and (b) Z-15, Z-25, and Z-Dol. The total lubricant film thicknesses on the disk surfaces are indicated. | |
3.3.2. Effect of Sliding
To account for the sliding results, we hypothesize that one possible action mechanism for chemical bonding of PFPE lubricants with a DLC surface is connected with the formation of radical-anion reactive intermediates, caused by the reaction of low-energy electrons (exoelectrons) emitted during the sliding process. The energy of exoelectrons isconsidered to be very low, of the order of 2-3 eV (Chaikin, 1967).
Step 1 Low-energy electron emission process (1-4 eV) during sliding
Step 2 Attachment of emitted electron to lubricant molecule
|
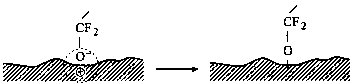
Step 3 Chemical reactions with the surface
| 
H• species may recombine and generate hydrogen molecules H2
or they can interact with the dangling bonds
| 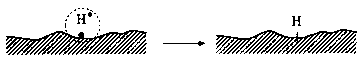
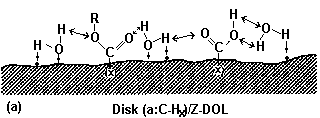
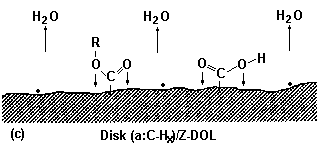
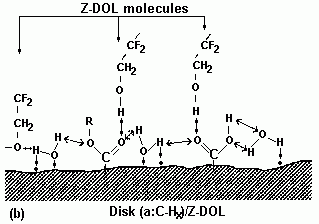
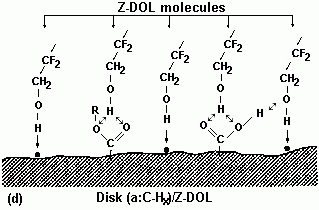
Fig. 7. Low-energy electron emission process during sliding on a Z-Dol lubricated surface and the interaction of emitted electrons with the lubricant molecules and subsequent interactions with surface. A simple dot represents the unpaired electron, known as a free radical. A free radical has no positive or negative charge but is very reactive because it is unpaired.
| |
|
|
that in Fig. 7. The low-energy electrons then attach to lubricant molecules and form the reactive species •CF2O¯ and •CF2O— (Step 2). The reactive group —CF2O¯ allows chemical reaction with the surface and forms bonded molecules —CF2—O— (Step 3). Radical species •CF2O— can react with the surface and form the dangling bond —CF2O— on the surface (Step 3). Free radicals •CF2O— can recombine, forming a PFPE molecule, or they can interact with Z-15 lubricant molecules, producing a smaller PFPE compound and generating another free radical [Z-15-F]•. The latter radical can recombine with a •CF2O— free radical, generating a higher molecular-weight lubricant compound.
4. conclusions
The chemical bonding of PFPE lubricants on a DLC surface can be enhanced by either prebake and/or sliding. For polar lubricants such as Z-Dol, prebake can activate the DLC surface and hence increases the access to lubricant molecules that can chemically bond on the surface. The prebaked DLC surface can increase the fraction of the bonded layer by a factor of 2. For polar and nonpolar PFPE lubricants including Z-Dol, Z-15, and Z-25, sliding at the interface can emit low-energy electrons. The electrons can attach to the lubricant molecules and allow the chemical reaction between lubricant molecules and a DLC surface. This enhances the chemical bonding.ACKNOWLEDGMENTS
Financial support for this study was provided by the industrial membership of the Computer Microtribology and Contamination Laboratory (CMCL).REFERENCES
- Bhushan, B. (1996), Tribology and Mechanics of Magnetic Storage Devices, 2nd ed., Springer-Verlag, New York.
- Chaikin, S. W. (1967), "On Frictional Polymer," Wear 10, 49-60.
- Kajdas, C. (1985a), "On Negative-Ion Concept of EP Action of Organo-Sulfur Compounds," ASLE Trans. 28, 21-30.
- Kajdas, C. (1985b), "About a Negative-Ion Concept of the Antiwear and Antiseizure Action of Hydrocarbons during Friction," Wear 101, 1-12.
- Kajdas, C. (1987), "About an Anionic-Radical Concept of the Lubrication Mechanism of Alcohols," Wear 116, 167-180.
- Nakayama, K., Yamanaka, K., Ikeda, H., and Sato, T. (1997a), "Friction, Wear, and Triboelectron Emission of Hvdrogenated Amorphous Carbon Films," Tribol. Trans. 40, 507-513.
- Nakayama, K., Bousaid, B.. and Ikeda, H. (1997b), "TriboElectromagnetic Phenomena ofHydrogenated Carbon Films-TriboElectrons," ASME J. Tribol. 119, 764-768.
- Vurens, G. H., Gudeman, C. S., Lin, L. J., and Foster, J. S. (1992), "Mechanism of Ultraviolet and Electron Bonding of Perfluoropolyethers," Langmuir, 8, 1165-1169.
- Zhao, Z., and Bhushan, B. (1996), "Effect of Bonded Lubricant Films on the Tribological Performance of Magnetic Thin-Film Rigid Disks," Wear 202, 50-59.
- Zhao, Z., Bhushan, B., and Kajdas, C. (1999). "Tribological Performance of PFPE and X-1P Lubricants at Head-Disk Interface. Part II. Mechanisms," Tribol. Lett. 6, 141-148.