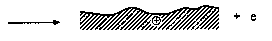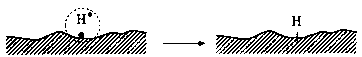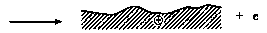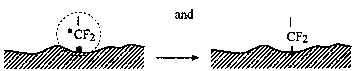Mechanism of Interaction and Degradation of Perfluoropolyethers with a DLC Coating in Thin-Film Magnetic Rigid Disks: A Critical Review
Czeslaw Kajdas¹ and Bharat Bhushan²
Abstract
This paper reviews, discusses, and analyzes the literature on the interaction and degradation mechanisms of perfluoropolyether lubricants with carbon protective overcoats used for magnetic media. Emphasis is placed on the degradation process of perfluoropolyether lubricants under sliding conditions. Detailed comments on various degradation mechanisms are presented. Particular stress is put on Z-DOL lubricant degradation mechanisms. It is believed that the dominant degradation mechanism is the low-energy electron initiated degradation mechanism.
* — Published in : J. Info. Storage Proc. Syst, Vol., 1, 1999
1. INTRODUCTION
The perfluoropolyether (PFPE) class of lubricants has been widely used for numerous special tribological applications because of their superb oxidative and thermal stability properties. The most sophisticated applications include magnetic recording disks for computer data storage (Bhushan, 1996). In computer industry applications, mostly for topical lubrication to reduce the wear of rigid disks, commonly used lubricants include Fomblin Z and Demnum, as well as their polar derivatives containing various reactive end groups, e.g., hydroxyl (Fomblin Z-DOL, Demnum SA), and piperonyl (Fomblin AM 2001; Bhushan, 1994).
The areal storage density, the number of bits stored per unit area, has been increasing at a rate of greater than 40% per year since 1990 (Simonds, 1995). This trend relates to a decrease in the flying height or spacing between the read-write head and the surface of the magnetic storage disk. Currently, the magnetic medium is protected with a hard overcoat, which is mostly a hydrogenated amorphous carbon film; however, nitrogenated carbon has been considered as well.
The primary goal of this review paper is to provide a better understanding of the PFPE lubricant interaction with carbon overcoats, under both static and sliding conditions. The secondary aim of the work is to elaborate and discuss in detail a model of polar lubricant-substrate interaction based on the hydrogen bonding. Emphasis is also placed on PFPE degradation mechanisms showing that low-energy electrons may play an important part in the PFPE degradation process.
2. PFPE LUBRICANTS
Perfluoroalhylpolyether (PFPE) lubricants are a group of polymers composed entirely of carbon, fluorine, and oxygen. Development of PFPE's as lubricants was first disclosed over 30 years ago (Gumprecht, 1966). Since that time, the following four major types of PFPE lubricants (Del Pesco, 1994), that is,
I
CF3—O—(CF2—CF2—O)m—(CF2—O)n—CF3
II
CF3—O—(CF—CF2—O)m—(CF2—O)n—CF3
║
CF3III
CF3—CF2—CF2—O—(CF2—CF2—O)m—CF2—CF3
IV
CF3—CF2—CF2—O—(CF—CF2—O)m—CF2—CF3
║
CF3
have become commercially available. By brand names they are known as Fomblin Z (I), Fomblin Y (II), Demnum (III), and Krytox (IV). They are colorless, odorless, compatible with most other materials, liquid over a wide temperature range, and completely inert to most chemical agents, including oxygen. The linear PFPE structures (I and III) show less change of viscosity with temperature and pressure when compared with nonlinear PFPE's (structures II and IV). Demnum (III) and Krytox (IV) contain one type of ether group and are homopolymers, whereas Fomblin Z (I) and Fomblin Y (II) contain two types of ether groups. PFPE lubricants are superior candidates to be used under severe and starved operating conditions, particularly at high speeds and elevated temperatures, as well as under heavy loads. For example, they are being developed as lubricants for special applications in such severe environments as in aerospace engines and satellite instruments (Jones and Snyder, 1980; Jones, 1995). Another specific application of PFPE lubricants relates to gas turbine engine oils (Gschwender et al., 1992). PFPEs are the current lubricants choice for magnetic recording media; however, constantly increasing recording densities and faster data transfer rates in the magnetic recording industry-apart from rising smoother thin-film disk surfaces and lower flying heights-require improved PFPE lubricants.
The best examples of the improved PFPE lubricants for magnetic recording media are Z-DOL and Demnum SA. Z-DOL includes two hydroxyl groups combined with the PFPE molecule by means of a —CH2 bridge. Demnum SA includes the —CH2—OH functional group only at one end of the PFPE molecule. PFPE lubricants without functional groups are nonpolar. Those lubricants with functional groups are becoming popular because the electrons of the functional group, for example, in Z-DOL, are not shared equally between the oxygen atom and hydrogen atom. This is because the oxygen atom draws electrons in a bond toward itself and thereby obtains a partial negative charge. To obtain balance, the hydrogen atom has a partial positive charge. Accordingly, the polarity of a compound relates to the magnitude of the separation of charge. As they have both positive and negative poles they are called dipole; that is, they have a dipole moment. Dipole moment is particularly important from the point of view of weak intermolecular bonds (van der Waals forces) in liquids. These forces include the following types of molecular interactions:
- dipole-dipole,
- dipole-induced dipole, and
- induced dipole-induced dipole.
The dipole-dipole molecular interaction clearly relates to the hydrogen bonding (hydrogen bridge formation) that can be found in both water and alcohols. Thus, hydrogen bonding seems to be of particular importance for the interaction of polar PFPE lubricants (mostly Z-DOL and Demnum SA) with a diamondlike carbon (DLC) coating and Al2O3-TiC head slider substrate in the interface. This especially relates to a humid environment because the PFPE hydroxyl (OH) group can easily form hydrogen bonds to neighboring water molecules. Such compounds, like water, are said to be hydrophilic. Accordingly, PFPE lubricants without specific functional groups including the -OH group are hydrophobic.
In the case of Z-DOL, a hydrogen atom, covalently bonded with an oxygen atom in the HO bond, has a partial positive charge on the end of the bond. This hydrogen atom can be easily attached to the negative charge of other molecules. Similarly, the lone pairs of electrons in the oxygen atom can be attached to positive charges of other molecules. A hydroxyl group (OH), as a part of an alcohol group in the Z-DOL molecule or water molecule structure, can form hydrogen bonds through its hydrogen atom and the unshared pairs of electrons on the oxygen atoms of neighboring molecules, resulting in an intrinsically polar molecule. The most recent work clearly demonstrated the importance of hydrogen bonding in the tribological performance of Z-DOL and X-1P lubricants at a head-disk interface (Zhao et al., 1999a). Major characteristics of these specific polymers along with other most important PFPE lubricants are shown in Table 1. Other functional groups of PFPE lubricants include carboxyl, ester, or piperonyl entity.
Table 1 Chemical Structure and Selected Properties of PFPE Lubricants
Lubricant Formula End Groups Molecular
Weight
(Daltons) Density
(x10³ kg/m³ at 20°C) Kinematic
Viscosity
(mm²/s)
Z-03 CF3—O—(CF2—CF2—O)m—(CF2—O)n—CF3 —CF3 3600 1.82 30 @ 20°C
Z-15 CF3—O—(CF2—CF2—O)m—(CF2—O)n—CF3 —CF3 9100 1.84 150 @ 20°C
Z-DOLa HO—CH2—CF2—O—(CF2—CF2—O)m—(CF2—O)n—CF2—CH2—OH ; (m/n ~ 2/3) —OH 2000 1.81 80 @ 20°C
34 @ 40°C
SAa F—(CF2—CF2—CF2—O)m—CF2—CF2—CH2—OH —OH 3600 1.87 75 @ 40°C
YR
CF3
║
CF3—O—(CF—CF2—O)m—(CF2—O)n—CF3
║
CF3 —CF3 6800 1.91 1600 @ 20°C
a — Functional or polar lubricants
The characteristic feature of all these functional groups is that they are polar. In other words, the polar groups determine the interaction level between a lubricant and various carbon surfaces. Usually, the magnitude of such interaction is evaluated by the heat of adsorption. Pertinent data are collected in Table 2 (from Yanagisawa, 1994), which shows heats of adsorption for PFPE with no functional group, ester, piperonyl, and hydroxyl groups to graphite, CVD carbon, sputtered carbon, amorphous carbon, and diamond. Table 2 clearly demonstrates the highest heat of adsorption for hydrophilic groups. This is the reason why Z-DOL and Demnum SA are currently the most-used lubricants to lubricate carbon overcoated disks. From the viewpoint of the functional group type, the magnitude of heat of adsorption is in the following order:
none < ester < piperonyl < hydroxyl
showing that the adsorption energy between functional groups and carbons increases with the increasing hydrophilic affinity of these groups. In contrast, the hydrophilic affinity of lubricants is also of importance for interaction of the environmental humidity with lubricant molecules. This relates to the hydrogen bonding of polar molecules in which there is a partial separation of charge to give positive and negative poles. It is of note at this point that hydrogen bonds may also be somehow separated from other examples of van der Waals forces because they are stronger than other intermolecular bonds. The strength of the hydrogen bond in water has been estimated to be between 10 and 12 kJ/mol (Bonder and Pardue, 1989). For comparison, the bond between planes of carbon atoms in a graphite structure is estimated to have a dissociation enthalpy of 17 kJ/mol.
Recently, cyclic phosphazenes have been developed as advanced lubricant additives for rigid thin
Table 2 Heat of Adsorption for PFPE's with Various Functional Groups to Various Carbon Surfaces
Adsorbent (Carbon) Functional Group (PEPE) Heat of Adsorption (mJ/m²)
Graphite No functional 0.00
Carbon
CVD No functional 0.01
Sputtered No functional -
Amorphous No functional 0.05
Diamond No functional 0.07
Graphite Ester 0.06
Carbon
CVD Ester 0.15
Sputtered Ester 0.32
Amorphous Ester -
Diamond Ester 0.55
Graphite Piperonyla 0.44
Carbon
CVD Piperonyla 1.5
Sputtered Piperonyla -
Amorphous Piperonyla 2.62
Diamond Piperonyla 2.57
Graphite Hydroxyl 3.56
Carbon
CVD Hydroxyl 5.64
Sputtered Hydroxyl 14.60
Amorphous Hydroxyl 19.14
Diamond Hydroxyl 38.47
a — Piperonyl is : 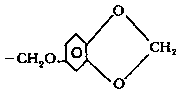
film magnetic media. As phophazenes have totally different chemical structure from PFPE they will be discussed separately in another review paper. It is only to note at this point that pertluoropolyether triazene and monophosphatriazene have been considered as
Fig. 1 Physical processes associated with surface deformation during boundary friction (Kajdas, 1997).
lubricant and additive for space application (Jones and Snyder, 1980; Jones, 1995) and for gas turbine engine oils (Gschwender et al., 1992), respectively. The characteristic feature for these two compounds is the presence of the six member ring including apart from phosphorus atoms 3 nitrogen atoms or 3 nitrogen atoms and one phosphorus atom (monophosphazene). The ring of the X-1P molecule is composed of nitrogen and phosphorus atoms. Such structures adsorb well on various substrates. The most recent papers (Zhao and Bhushan, 1999; Zhao et al., 1999a; Kasai, 1999) describe not only X-1P's role as PFPE lubricant in disk files, but also discuss its lubrication mechanism.
3. CARBON OVERCOAT AND ITS INTERACTION WITH PFPE'S
3.1. Introduction
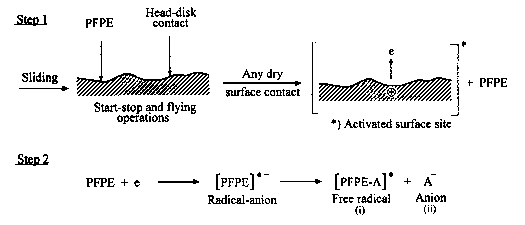
Head-disk interface interactions for a given tribological system are controlled by the chemistry of both head and disk surfaces as well as by the lubricant chemistry. In a magnetic rigid-disk drive, recording or playback is accomplished by relative motion between a magnetic medium against a stationary read-write magnetic-head slider. There is a physical contact between the slider and medium during starting and stopping. To reduce the wear generated during the contact-start-stop (CSS) operation, hard overcoats and liquid lubricant films are typically applied to the disk surface. In modern high-end disk drives, the head-to-medium separation is of the order of 25 nm, and surfaces have a roughness of the order of 1.5 nm rms. The need for increasing high recording densities requires that surfaces should be as smooth as possible and flying heights be as low as possible. In contrast, smoother surfaces lead to increase in adhesive interaction between the mating surfaces controlling both friction and interface temperatures. It should also be emphasized that closer flying heights lead to occasional rubbing of high asperities and increased wear, accompanied with various physical processes. Usually, the application of mechanical energy associated with friction releases a great number of physical processes (Fig. 1), which can be the cause of tribochemical reactions of solids with lubricants molecules. According to Kajdas (1987, 1994), the most important factor governing tribochemical reactions under boundary friction is associated with the action of low-energy electrons with lubricating oil components. This is the basis of the negative-ion-radical action mechanism (NIRAM).
In order to find out if electrons are emitted at head-disk sliding contacts in magnetic recording media, electron emission phenomena were investigated together with the friction and wear in a frictional system of an alumina ball sliding on amorphous and hydrogenated amorphous carbon films in a dry air atmosphere (Nakayama and Ikeda, 1996). The researchers found that electrons are emitted during wear of both amorphous and hydrogenated amorphous carbon films. The transition from low to high electron emission intensity was caused by the increase in wear of the carbon films.
The general model of NIRAM assumes the creation of two types of activated sites on friction surfaces, i.e., thermally activated sites and sites activated by a low-energy electron (exoelectron) emission process. The situation is illustrated in Fig. 2. Bearing in mind that thin films of perfluoropolyethers can be attached to a variety of substrates, such as amorphous carbon, silica, and gold, by illumination of the substrate with 185-nm UV light (Saperstein and Lin, 1990; Vurens et al., 1992), it is possible to hypothesize that lubricant reactions with head-disk sliding contacts might not only be initiated by temperature and/or catalysis, but
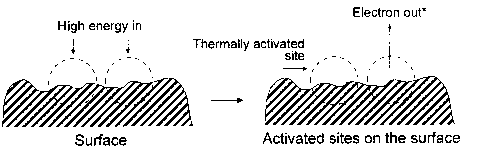
Fig. 2 Surface activation during friction (Kajdas, 1994).
also by low-energy electrons emitted from the carbon substrate under the boundary friction conditions. The hypothesis can be strengthened by the fact that the electron bonding is some 3 to 4 orders of magnitude more efficient than the UV bonding process (Vurens et al., 1992). Application of the NIRAM approach to the head-disk sliding contact tribochemistry will be described in a later subsection.
Currently, carbon films lubricated with PFPE are widely used as the protective coating for thin-film magnetic storage disks. Therefore, before the work on PFPE degradation-reaction mechanisms is reviewed; carbon overcoats will be discussed.
3.2. Carbon Overcoat
3.2.1. Chemical
Carbon is known to occur in various elemental forms. There are three allotropic crystalline forms (diamond, graphite, and fullerenes) and a number of amorphous (noncrystalline) forms. The amorphous carbon form encompasses carbon black, charcoal, and coke. The properties of diamond are particularly noteworthy. It is among the least volatile substances known, and its melting temperature exceeds 3550 °C. This specific allotropic carbon is also the hardest substance known, and it expands less on heating than any other material. These diamond properties are a logical consequence of the fact that carbon atom forms covalent bonds (sp³) to four neighboring carbon atoms arranged toward the corners of tetrahedron. The strength of the C-C bonds plus their arrangement in space provide the unusual properties of diamond. Diamond is an excellent insulator, opposite to graphite. The specific feature of amorphous carbons relates to the fact that they usually have unpaired electrons (dangling bonds).
For rigid-disk overcoats, mostly hydrogenated carbon of various amorphous forms a-C:H are used. Hydrogenated carbon films generally have higher resistivity and therefore are expected to be more corrosion resistant to galvanic corrosion. Hydrogen content significantly influences various properties of the carbon films. Bhushan (1996, 1999a) reviewed DLC film applications for magnetic media, considering their physical nature, chemical structure, and tribological characteristics. It has also been emphasized that many carbons deposited by sputtering and other deposition techniques for nondisk applications have been found to be possibly more diamondlike; however, sputtered carbon for thin-film disks tend to be less diamondlike (primary amorphous), with few graphitic and diamond crystallites. Lee, Smallen, et al. (1993), investigating hydrogenated carbon films, found that the carbon bonding character changes from sp² to sp³ with an increase of hydrogen content. Dillon et al. (1984) revealed that the number or size of the carbon microstructure decreases with an increase in the hydrogen content. Most interestingly, the amorphous carbon film structures change from graphitelike structures with more sp² bonding when there is no hydrogen content, via diamondlike structures with more sp³ bonding when there is intermediate hydrogen content, to polymerlike structures with —CH2 bond when there is excessive hydrogen content (Dillon et al., 1984; Cho et al., 1990). These structural changes also cause the change in the internal stress of the hydrogenated carbon films.
Perry and Somorjai (1995), investigating the adsorption of water and small perfluorinated compounds on hydrogenated amorphous carbon films (a-C:H), found a relationship of hydrogen content on the surface free energy. Changes in hydrogen content of the films were observed to modify the a-C:H surface free energy and to directly influence the desorption temperature of submonolayer coverages of both water and perfluorinated species. They concluded that hydrogenated carbon films exhibiting the highest surface free energy provided a greater attractive interaction for the model lubricants and may provide greater stability of thin lubricant films on these surfaces.
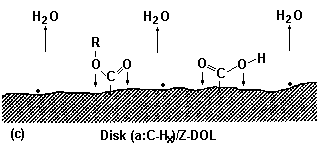
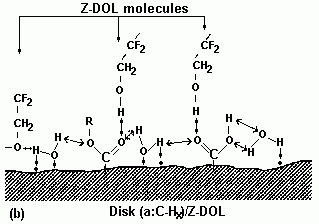
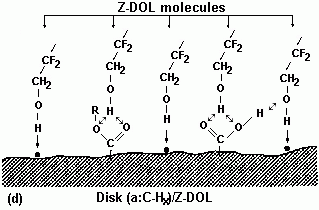
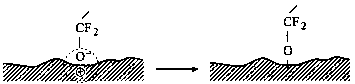
Carbon surface chemistry of magnetic disks is of particular importance from the viewpoint of lubricant interaction with the disk or head surface. A variety of functional groups has been found to exist on carbon surfaces (Silva et al., 1992). Yanagisawa (1994), using X-ray photoelectron spectroscopy, determined many oxygen-containing groups along with the conjugated group on the sputtered carbon surface. Table 3 (Yanagisawa, 1994) summarizes the ratio of these groups to total carbon. From the measured functional groups and ESR spectrometry measurement results, which provided evidence for the existence of dangling bonds on the amorphous carbon, Yanagisawa (1994) proposed an amorphous carbon surface model (Fig. 3). The model particularly emphasizes the presence of dangling bonds (three dots shown in Fig. 3); five oxygen-containing functional groups (hydroxyl, carbonyl, carboxyl, oxide, and ester) are included by only one group each.
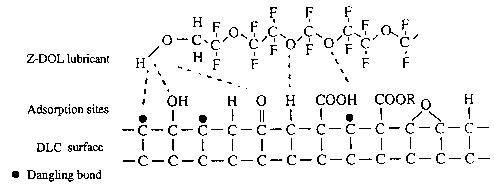
Fig. 3 Diagram for the Z-DOL molecule adsorbed on the carbon surface at 20 °C (Yanagisawa, 1994; Bhushan and Zhao, 1999).
Table 3 Functional Group to Total Carbon Ratio on Hydrogenated DLC Surfaces and Their ICN's
Functional Group Formula % Total Carbon ICN
Ether —C—O—C— 15.7 20
Carbonyl ═C═O 6.0 65
Ester —COOR 3.5 60
Carboxyl —COOH 0.5 150
Hydroxyl —COH 0.3 100
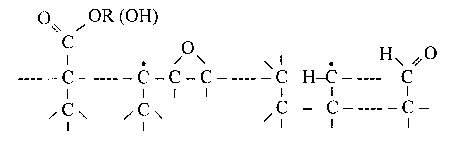
Fig. 4 Simplified diagram of the chemical structure of a hydrogenated carbon overcoat surface.
The ratio of oxygen-containing functional groups presented in Fig. 3 does not relate to their percentage of total carbon. Even considering inorganic character numbers (ICN), as discussed by Yanagisawa (1994), the most abundant functional groups would relate to the ether group (15.7% of total carbon with ICN = 20), and the carbonyl group (6.0% of total carbon and ICN = 65). The presence of other oxygenated groups (—COOR, —COOH, and —OH) is much less significant, although their ICN's are high, particularly for the carboxyl group (ICN =150). The inorganic character number shows the relative magnitude of association between function groups.
Taking into consideration all the above discussed data, we propose the use of a simplified carbon surface diagram (Fig. 4) which reflects the real content of oxygenated functional groups as presented in Table 3. The simplified diagram shows only one combined group, representing both the ester group and the carboxyl group. As the carbonyl group cannot be combined with a five-valence carbon (see Fig. 3), in Fig. 4 it is presented in the form of an aldehyde group, which is substantiated by the fact that mostly carbon overcoats are hydrogenated. For comparison, Fig. 5 shows an idealized graphite surface layer plane with various functional groups located at the periphery of the plane (Janzen, 1982).
Carbon overcoats lubricated with PFPE are used as the protective coating for magnetic storage disks and metal evaporated (ME) tapes. The thickness of the protective overcoat is 10-15 nm for disks and 8-10 nm for tapes (see Fig. 6). The addition of a limited amount of hydrogen in the amorphous carbon overcoat improves the CSS durability of thin-film disks lubricated with PFPE's.

Fig. 5 Aromatic layer plane with functional side groups (Janzen, 1982).
Hydrogen incorporated in the amorphous carbon changes its chemical and physical properties. For example, electrical resistance and hardness of the carbon film vary with the amount of hydrogen. A hydrogenated carbon surface chemistry differs significantly from a nonhydrogenated one (Wang et al., 1996). The same is true for the triboemission process (Nakayama, Bou-Said, et al., 1997). However, it is not due to dangling bonds. Yanagisawa(1994) discussed the question why dangling bonds are not saturated by hydrogen, referring to papers by Wada et al. (1980). The authors of the two latter papers emphasized that dangling bonds in carbon are relatively stable. Dangling bonds are not easy to bond to hydrogen because double bonds in sp² carbon would be saturated first with hydrogen (Cho et al., 1990). According to Jansen et al. (1985), even if the carbon is hydrogenated, the density of unpaired
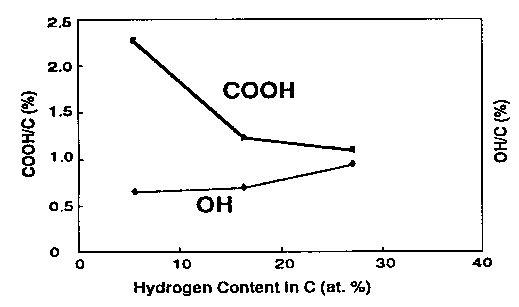
Fig. 7 Concentration of carbonyl and hydroxyl end groups of a-C:H films (Wang et al., 1996).
electrons (dangling bonds) in the hydrogenated carbon decreases only from 1018 to 1016 cm -3.
Interestingly, Wang et al. (1996) found that the concentration of the carboxyl groups on carbon surface decreases with increasing hydrogen content, whereas that of the hydroxyl groups exhibits an increasing tendency dependence on the hydrogen content increase. This specific relationship is depicted in Fig. 7.
Another characteristic feature of hydrogenated carbons concerns triboelectromagnetic phenomena. These phenomena include the microplasma formation of sliding contacts, the triboemission of electrons, ions, and photons, and tribocharging (Nakayama, Bou-Said, et al., 1997; Nakayama, Yamanaka, et al., 1997). As already mentioned in the preceding subsection, Nakayama and Ikeda (1996) first investigated the emission of triboelectrons, using the frictional system of alumina sliding on hydrogenated carbon films with different hydrogen content. Alumina simulated the Al2O3-TiC head slider. They presented evidence that the intense electron emissions were controlled by the hydrogen content.
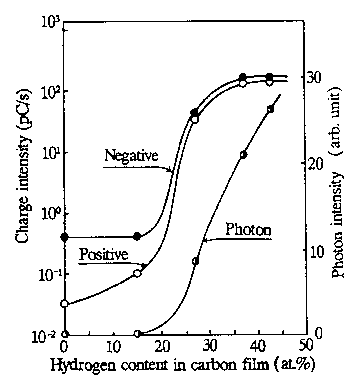
In the most recent work by Nakayama, Bou-Said, et al. (1997), triboemission of negatively charged particles and positively charged particles, tribocharging, and friction coefficient were measured simultaneously by using a tribosystem with diamond sliding on hydrogenated carbon films in ambient air. The hydrogen content of the carbon films differed from 0 to 43 at. %. Figure 8 clearly demonstrates that all the carbon films tested emitted both negatively and positively charged particles under the sliding conditions used. A typical pin-on-disk apparatus was used in which the diamond stylus was a cone having an included angle of 90° and a tip radius of 300 µm. Sliding experiments were conducted under an applied normal force of 200 mN; the wear track diameter was 16.5 mm and the sliding velocity was 5.2 cm/s. Tests were carried out in ambient air that had a relative humidity of 52-53%. Interestingly, at low hydrogen content (below 15 at. %), no tribophotons were observed.
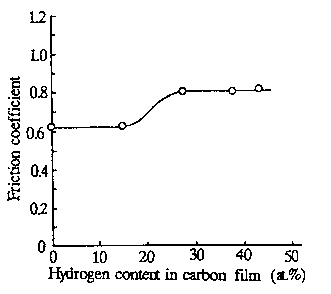
The emission intensities of the negatively charged particles and positively charged particles are low in that region of hydrogen content. Figure 8 also manifests that the charge intensity of negative particles is greater than that of positive particles, particularly in the low hydrogen content region. In the region of hydrogen contents between 15 at. % and 37 at. %, the emission intensity of the charged particles increased very rapidly, accompanied by the photon emission. It is of note at this point that the friction coefficient also increased from a low to a higher value in the region of hydrogen content from 15 to 27 at. %. The relationship of the friction coefficient values and hydrogen content in the carbon films tested is presented in Fig. 9. From these results, Nakayama, Bou-Said, et al. (1997) concluded that a microplasma state is formed at the frictional contacts of diamonds sliding on hydrogenated carbon films.
Other research by Wang et al. (1995) studied the effect of hydrogen content on the tribology of the head-disk interface. Among other things, they found that the durability of the film increased as the hydrogen content was increased from 12 to 36 at. %.
3.2.2. Interaction Mechanisms of a Carbon Overcoat with PFPE Lubricants
There is a wide variety of issues, combined with a possibility of obtaining adequate lubrication and protection of the disk surface. One is the PFPE lubricant thickness and its bondability with the carbon surface. A second is the chemical stability of the lubricant film under sliding conditions. Another issue is the ability of the lubricant to flow across the surface. While it must have an interaction with the DLC (a-C:H) overcoat that is strong enough to prevent loss during spinning, it must at the same time be able to flow into small regions of the surface that are depleted of lubricant as a result of contact with the head. Unhydrogenated amorphous carbon has a small energy gap and thus has a relatively high electrical conductivity. The small energy gap results possibly because of increased presence of sp² bonds. Resonance hybridization leads to delocalization of the electrons within a plane. Because electrons are free to move through the plane, they can conduct an electric current. Such material usually develops dangling bonds. Kaplan et al. (1985) found, however, that the hydrogenated amorphous carbon continues to have a loss energy gap of 1.2 eV in comparison with the direct gap of purely sp² bonded carbon (7.3 eV). This led to the conclusion that the presence of sp² bonding states requires the hydrogen not only to passify the dangling bond but also to saturate the double graphite bonds (sp²). Thus, for a-C:H films, it is of importance to know to what extent hydrogen passifies the dangling bonds and how these control the carbon surface activity and electrical conductivity. This issue also interrelates with electron emission intensity during sliding, as presented in Fig. 8. It is also pointed out that the electrical conductivity and hardness have been found to depend on the carbon preparation conditions (Enke, 1981; Cho et al., 1990). Amorphous hydrogenated carbon films of high electrical resistivity were studied by Miller and McKenzie (1983), using electron spin resonance both as prepared and after vacuum heat treatment. They found that all the carbon films investigated had a high spin density in the range from 10²¹ to 10²² spins/kg. Dangling bonds in a-C:H overcoats should be of particular importance from the viewpoint of the carbon overcoat interaction with PFPE lubricants. Most interestingly, for a-C:N materials, Torng and Silvnrtsen (1990) found that the energy gap is increased from 1.1 to 1.4 eV when the nitrogen partial pressure increased from 2.5 mTorr to 10 mTorr. These values are very close to the energy gap value (1.2 eV) for hydrogenated amorphous carbon (Kaplan et al., 1985). At the moment we have not found any information on triboemission from the a-C:N substrate; however, comparing the energy gap data for both a-C:H and a-C:N materials, we can speculate that nitrogenated carbon also emits electrons under boundary friction conditions, either in contact with diamond, alumina, or any DLC substrate. As with a 2.5-mTorr N2 partial pressure in the plasma, the nitrogen content in the carbon is already quite high (25 at. %; Torng and Silvertsen, 1990); we can assume that lower nitrogen content a-C:N substrates emit fewer electrons than a-C:H substrates. Considering the interaction of lubricants with surfaces of hydrogenated carbon films, we must bear in mind what types of active sites exist on the surface (see Figs. 3 and 4) and how they can interact with the lubricant molecule. Thus, the most important factor controlling this interaction will relate to the chemistry of the PFPE end groups. Accordingly, lubricants with polar end groups such as hydroxyl, carboyl, or piperonyl can strongly interact with the DLC surface, forming bonded films. To achieve necessary wear protection, the magnetic medium is covered with a film (10-20 nm; see Fig. 6 ) overcoat of a wear-resistant amorphous hydrogenated carbon, and it is coated with a molecularly thin film (1-2 nm) of a perfluoropolyether lubricant.
Perry and Somorjai (1995) investigated the interaction of low-molecular model lubricants with the surfaces of a-C:H carbon films. To evaluate the interaction of model perfluorinated lubricants with thin a-C:H films, they employed thermal desorption spectroscopy (TDS). The following compounds were tested:
- perfluorodiethyl ether,
- perfluoropentane,
- perfluoro-octane, 2,2,2-trifluoroethanol, and 1,1, 7-H-perfluoroheptanol.
They found that all the tested molecules, modeling both the backbone and end groups, reversibly adsorbed with little chemical reaction with the a-C:H surface. Lee, Zube, et al. (1993) postulated that end-group chemistry dominates the adsorption process of the lubricant and provides an anchor to the surface. Perry and Somorjai (1995) have not found any evidence for chemical bonding of the alcohol end group to the carbon surface. In contrast, they emphasized that studies of the desorption of monodispersed fractions of Z-DOL lubricant demonstrated that desorption begins in the temperature range of decomposition, suggesting that physisorption energies are sufficient in bonding the lubricant to the surface at temperatures less than 377° C. Increases in desorption energies were observed with increasing chain length and classified as purely van der Waals interactions. Incorporation of alcoholic end groups promoted hydrogen bonding to the surface and produced about a 20 kJ/mol increase in desorption energy relative to that of a perfluorinated alkane of similar chain Length. Ether linkages within the model lubricant promoted little increase, as fluorine substituents effectively screen the oxygen. Waltman et al. (1998) showed that the evaporation of Z-DOL lubricant from the disk has an activation energy of 5.4 kcal/mole; Z-DOL bonding to the disk is relatively easy with an activation energy of 3.6 kcal/mole. Evidence for Z-DOL lying flat 3n the carbon surface is obtained from the thickness dependence of the dispersive component of the surface energy for both mobile and bonded Z-DOL. The major interactions leading to this conformation are between the oxidized sites on the carbon and the hydroxyl end groups of the lubricant. They also indicated that the model reaction between Z-DOL and the carboxylic acid terminated PFPE lubricant (Z-DIAC) raises the possibility that esterification reaction to form a chemisorbed lubricant may occur at elevated temperature.
Hara et al. (1991) investigated the bonding feature among a carbon overcoat film, atomic level contaminations, and perfluoropolyether lubricant with thermal energy (activation energy) as a parameter. They found that thermal energy significantly affects the bonding feature of the lubricant; however, no explanation of the mechanism was provided. The emphasis was made on the fact that removal of the contamination reduced the coefficient of friction and increased media durability. Z-DOL containing hydroxyl groups strongly interacts with dangling bonds and functional groups on the carbon surface, apart from the overcoat interaction with the lubricant ether groups. Additionally, hydroxyl groups of the Z-DOL molecule can form hydrogen bonding with oxygen and hydrogen atoms of the carbon surface film functional groups. The most recent work (Zhao et al., 1999a) presents interaction details of Z-DOL lubricant and adsorbed water film on the head-disk interface. It is also emphasized that heating enhances the bonded Z-DOL film significantly. It has also been shown that prebaking at 150 °C doubles the bonded film of Z-DOL lubricant (Zhao et al., 1999b). This effect is accounted for in terms of the water molecules' removal from the carbon overcoat, thereby exposing more dangling bonds and the surface functional groups to an interaction with the lubricant molecules.
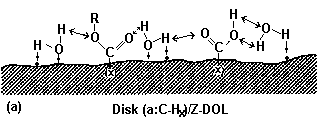
A schematic presentation of this effect is depicted in Fig. 10 (Zhao et al., 1999b).
4. DEGRADATION MECHANISMS OF PFPE LUBRICANTS
4.1. Introduction
A PFPE lubricant deposited on thin hard carbon coatings under static conditions interacts with the surfaces mostly through physical adsorption forces. The physically adsorbed lubricant film can, however, also be chemically bonded by a specific treatment of the deposited film. The known approaches to produce chemically bonded films encompass exposure of the lubricated disk to specific radiation modes, such as
- low-energy X-ray (Heideman and Wirth, 1984),
- nitrogen plasma (Homola et al., 1990),
- high-energy ion beam (Lee, 1991), and
- low-energy electrons and far ultraviolet (Vurens et al., 1992).
The effect of heating on Z-DOL bonding on carbon overcoats was discussed in subsection 3.2.2. It should be emphasized at this point, however, that the length of the thermal treatment and the temperature for PFPE lubricants with polar end groups are important factors in determining the bonding level (adsorption strength) of lubricants to the disk surface (Zhao and Bhushan, 1996).
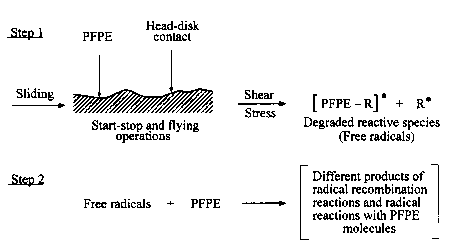
During sliding both physically adsorbed and chemisorbed PFPE lubricant molecules undergo degradation. The degradation process is very complex. Although there are a number of papers aiming at accounting for the PFPE degradation mechanism (e.g., see Zhao et al., 1999c), it is still not yet fully understood. Broad, detailed, and sophisticated research resulted in the development of several approaches. They include
- thermal decomposition processes,
- catalytic degradation processes,
- electron mediated degradation, and
- mechanical degradation by a shearing process.
The following subsections will discuss all these approaches to the PFPE degradation mechanism.
4.2. Thermal Decomposition
PFPE's as a class of fluid lubricants exhibit excellent thermal and oxidative stability. Notwithstanding the fact that they are thermally stable up to 350 °C (Helmick and Jones, 1990), there is clear evidence that their actual thermal stability is adversely affected by the presence of metals. Jones et al. (1983) demonstrated the effects of steel and some titanium alloys and additives on the thermal stability of linear perfluoroalkyl ether fluids. The presence of metal alloys drastically increases the rate of degradation. Two inhibitors, a perfluoroalkyl ether substituted monophospha-s-triazine and a perfluorophenylphosphine, were found to be highly effective in degradation reduction at 288 °C, but they had only limited effectiveness at 316 °C. Results of another work (Zehe and Faut, 1989) showed that complete degradation of Fomblin Z takes place at 185 °C in the presence of iron oxide (Fe2O3).
Interestingly, such degradation was not observed with the Krytox lubricant. The complete degradation of Fomblin Z was ascribed to the presence of acetal groups (—O—CF2—O—) in the polymeric chain, as suggested earlier by Jones et al. (1983).
Under sliding conditions, particularly at high sliding speeds, the temperature on hard carbon coatings can be sufficiently high to initiate PFPE thermal degradation (Bhushan, 1992). For instance, Huu et al. (1997), investigating friction and wear of hard carbon coatings at sliding speeds in the range 30-35 m/s in air, presented evidence that the temperature increase produced by friction in the contact zone was high, about 285 °C. The transition from the sp³ to sp² phase occurs at this temperature. As the transition consumes a large part of the friction energy in the contact, apart from creating a thin graphite layer on the track, it is also reasonable to suggest that in such a specific situation the thermal degradation of PFPE lubricant can take place. A schematic presentation of this most simple free-radical PFPE degradation mechanism is depicted in Fig. 11.
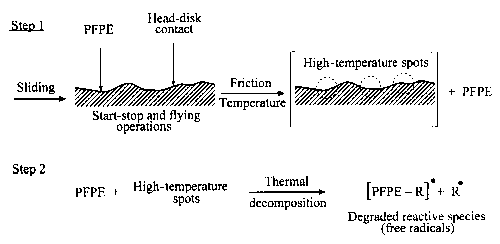
Fig. 11. Thermal degradation process of PFPE lubricants (temp. >350 °C); the free radicals can react with PFPE molecules and oxygen molecules, recombine with other free radicals, or decompose.
The presence of oxygen in the environment will lead to the thermal oxidative degradation process in which a very reactive peroxide radical can be generated.
4.3. Catalytic Degradation
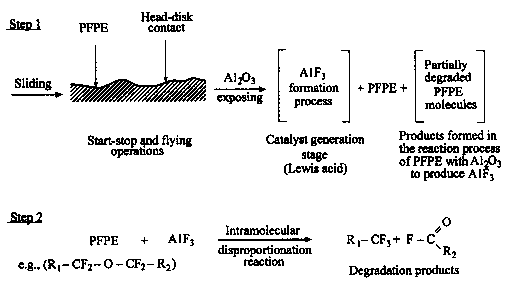
As a-alumina exists as the structural element in the construction of a head slider for a magnetic disk drive, the degradation mechanism of PFPE's by a-alumina was examined (Kasai et al., 1991). The results obtained led to a followup experiment of the degradation process in the presence of AlCl3, and at higher temperatures (Kasai et al., 1991; Kasai, 1992). Although a-alumina is a typical Lewis acid, A1C13 is a stronger Lewis acid. According to Kasai (1999), the above-mentioned studies conclusively demonstrated that the degradation of PFPE's in the presence of Lewis acids (metal oxides or halides) is singularly dominated by the intramolecular disporportionation reaction,
R1—CF2—O—CF2—O—CF2—R2
→ R1—CF3 + FC(O)—R2
where R1 is the left part of Z-DOL molecule from the above-shown acetal sector, and R2 is the right portion of Z-DOL molecule.
This reaction occurs most readily at acetal sector (O—CF2—O), but, given sufficient activation energy or in the presence of a stronger Lewis acid, it also occurs at other linkages. It was also found that, when the reaction occurred at the terminal linkage, the fluorine transfer was always from the terminal group into the internal sector (Kasai, 1999).
The degradation mechanism of a Z-type lubricant in the presence of alumina has been characterized as follows (Kasai et al., 1991):
- it is catalyzed by A1F3 formed during the induction period;
- it uniquely involves the acetal moiety (—O—CF2—O) of the polymer chains;
- it generates methoxy end groups (—O—CF3), and
- it results in fragmentation of polymer chains but does not involve an unzipping process.
Figure 12 shows schematically the reaction mechanism of the catalyzed degradation mechanism of a Z-type lubricant. The reaction sequence is initiated by a bidentate linkage between an acidic aluminum on A1F3 and the two oxygen atoms of an acetal unit, as presented in the upper part of Fig. 12.
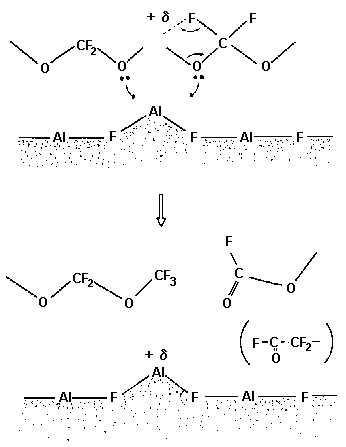
Fig. 12. Interaction between a Lewis acid site on AlF3 and an acetal sector of Z-lube (Kasai et al., 1991).
The partial positive charge developed at the acetal carbon induces a fluorine atom transfer from the adjacent CF2 unit leading to chain scission with transformation of the acetal unit into a methoxy (—O—CF3) end group, and the adjacent unit into either a fluoroformate F—CO—O—CF2 end group or an acylfluoride F—CO—CF2 end group from the adjacent unit, depending upon whether the adjacent unit was originally a methylene oxide unit or an ethylene oxide unit (Kasai et al., 1991).
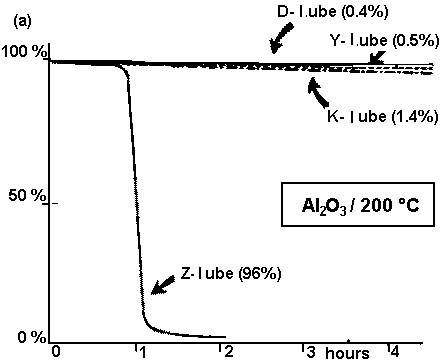
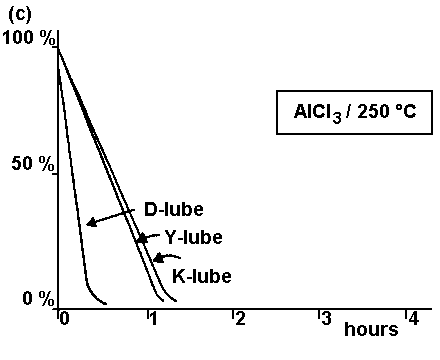
The catalytic degradation mechanism of PFPE lubricants is very well elaborated and clearly presented in the papers discussed above. Illustrative weight loss of all major PFPE lubricant types during heating in the presence of various catalysts, depicted in Fig. 13,
provides excellent information concerning the thermocatalytic stability of these lubricants. The degradation was induced by placing 5 g of lubricant and 1 wt. % of Al2O3 (or AlCl3) in a test tube, and immersing the tube in an oil bath maintained at a desired temperature. For each lubricant, the weight loss was measured after the heat treatment for a given period of time (Kasai, 1999). Looking at the modeled degradation processes presented in Fig. 13, from the viewpoint of the head-disk lubricated situation, we see that Fig. 13 (a) seems to be the most relevant to the real world. Under these test conditions, Al2O3/200°C all the investigated lubricants but the Z-type lubricant are very stable. In contrast, even the least stable lubricant starts to degrade after 50 min of heating. At this point the question arises whether such a situation can be attained under CSS sliding and flying conditions. We do not think so, as the test conditions for their experiments are extreme and cannot be believed to be achieved at a head-disk interface. However, note that Kasai (1999) reported some similarities between PFPE lubricant fragments released from the head-disk interface with volatile products generated from Z-type lubricants undergoing an alumina-catalyzed degradation process at 200 °C and head-disk interface studies conducted in UHV.
2. PFPE LUBRICANTS
Perfluoroalhylpolyether (PFPE) lubricants are a group of polymers composed entirely of carbon, fluorine, and oxygen. Development of PFPE's as lubricants was first disclosed over 30 years ago (Gumprecht, 1966). Since that time, the following four major types of PFPE lubricants (Del Pesco, 1994), that is,| I |
CF3—O—(CF2—CF2—O)m—(CF2—O)n—CF3
| II
|
CF3—O—(CF—CF2—O)m—(CF2—O)n—CF3 | CF3 III
|
CF3—CF2—CF2—O—(CF2—CF2—O)m—CF2—CF3
| IV
|
CF3—CF2—CF2—O—(CF—CF2—O)m—CF2—CF3 | CF3 |
- dipole-dipole,
- dipole-induced dipole, and
- induced dipole-induced dipole.
Table 1 Chemical Structure and Selected Properties of PFPE Lubricants | |||||||
| Lubricant | Formula | End Groups | Molecular Weight (Daltons) | Density (x10³ kg/m³ at 20°C) | Kinematic Viscosity (mm²/s) | ||
| Z-03 | CF3—O—(CF2—CF2—O)m—(CF2—O)n—CF3 | —CF3 | 3600 | 1.82 | 30 @ 20°C | ||
| Z-15 | CF3—O—(CF2—CF2—O)m—(CF2—O)n—CF3 | —CF3 | 9100 | 1.84 | 150 @ 20°C | ||
| Z-DOLa | HO—CH2—CF2—O—(CF2—CF2—O)m—(CF2—O)n—CF2—CH2—OH ; (m/n ~ 2/3) | —OH | 2000 | 1.81 | 80 @ 20°C 34 @ 40°C | ||
| SAa | F—(CF2—CF2—CF2—O)m—CF2—CF2—CH2—OH | —OH | 3600 | 1.87 | 75 @ 40°C | ||
| YR |
║ CF3—O—(CF—CF2—O)m—(CF2—O)n—CF3 CF3 | —CF3 | 6800 | 1.91 | 1600 @ 20°C | ||
| a — Functional or polar lubricants | |||||||
none < ester < piperonyl < hydroxyl
showing that the adsorption energy between functional groups and carbons increases with the increasing hydrophilic affinity of these groups. In contrast, the hydrophilic affinity of lubricants is also of importance for interaction of the environmental humidity with lubricant molecules. This relates to the hydrogen bonding of polar molecules in which there is a partial separation of charge to give positive and negative poles. It is of note at this point that hydrogen bonds may also be somehow separated from other examples of van der Waals forces because they are stronger than other intermolecular bonds. The strength of the hydrogen bond in water has been estimated to be between 10 and 12 kJ/mol (Bonder and Pardue, 1989). For comparison, the bond between planes of carbon atoms in a graphite structure is estimated to have a dissociation enthalpy of 17 kJ/mol.
Table 2 Heat of Adsorption for PFPE's with Various Functional Groups to Various Carbon Surfaces | |||||
| Adsorbent (Carbon) | Functional Group (PEPE) | Heat of Adsorption (mJ/m²) | |||
| Graphite | No functional | 0.00 | |||
| Carbon | |||||
|
| No functional | 0.01 | |||
|
| No functional | - | |||
|
| No functional | 0.05 | |||
| Diamond | No functional | 0.07 | |||
| Graphite | Ester | 0.06 | |||
| Carbon | |||||
|
| Ester | 0.15 | |||
|
| Ester | 0.32 | |||
|
| Ester | - | |||
| Diamond | Ester | 0.55 | |||
| Graphite | Piperonyla | 0.44 | |||
| Carbon | |||||
|
| Piperonyla | 1.5 | |||
|
| Piperonyla | - | |||
|
| Piperonyla | 2.62 | |||
| Diamond | Piperonyla | 2.57 | |||
| Graphite | Hydroxyl | 3.56 | |||
| Carbon | |||||
|
| Hydroxyl | 5.64 | |||
|
| Hydroxyl | 14.60 | |||
|
| Hydroxyl | 19.14 | |||
| Diamond | Hydroxyl | 38.47 | |||
| a — Piperonyl is : | 
| ||||
film magnetic media. As phophazenes have totally different chemical structure from PFPE they will be discussed separately in another review paper. It is only to note at this point that pertluoropolyether triazene and monophosphatriazene have been considered as
 | Fig. 1 Physical processes associated with surface deformation during boundary friction (Kajdas, 1997). |
lubricant and additive for space application (Jones and Snyder, 1980; Jones, 1995) and for gas turbine engine oils (Gschwender et al., 1992), respectively. The characteristic feature for these two compounds is the presence of the six member ring including apart from phosphorus atoms 3 nitrogen atoms or 3 nitrogen atoms and one phosphorus atom (monophosphazene). The ring of the X-1P molecule is composed of nitrogen and phosphorus atoms. Such structures adsorb well on various substrates. The most recent papers (Zhao and Bhushan, 1999; Zhao et al., 1999a; Kasai, 1999) describe not only X-1P's role as PFPE lubricant in disk files, but also discuss its lubrication mechanism.
3. CARBON OVERCOAT AND ITS INTERACTION WITH PFPE'S
3.1. Introduction
Head-disk interface interactions for a given tribological system are controlled by the chemistry of both head and disk surfaces as well as by the lubricant chemistry. In a magnetic rigid-disk drive, recording or playback is accomplished by relative motion between a magnetic medium against a stationary read-write magnetic-head slider. There is a physical contact between the slider and medium during starting and stopping. To reduce the wear generated during the contact-start-stop (CSS) operation, hard overcoats and liquid lubricant films are typically applied to the disk surface. In modern high-end disk drives, the head-to-medium separation is of the order of 25 nm, and surfaces have a roughness of the order of 1.5 nm rms. The need for increasing high recording densities requires that surfaces should be as smooth as possible and flying heights be as low as possible. In contrast, smoother surfaces lead to increase in adhesive interaction between the mating surfaces controlling both friction and interface temperatures. It should also be emphasized that closer flying heights lead to occasional rubbing of high asperities and increased wear, accompanied with various physical processes. Usually, the application of mechanical energy associated with friction releases a great number of physical processes (Fig. 1), which can be the cause of tribochemical reactions of solids with lubricants molecules. According to Kajdas (1987, 1994), the most important factor governing tribochemical reactions under boundary friction is associated with the action of low-energy electrons with lubricating oil components. This is the basis of the negative-ion-radical action mechanism (NIRAM).
 |
| Fig. 2 Surface activation during friction (Kajdas, 1994). |
3.2. Carbon Overcoat
3.2.1. Chemical
Carbon is known to occur in various elemental forms. There are three allotropic crystalline forms (diamond, graphite, and fullerenes) and a number of amorphous (noncrystalline) forms. The amorphous carbon form encompasses carbon black, charcoal, and coke. The properties of diamond are particularly noteworthy. It is among the least volatile substances known, and its melting temperature exceeds 3550 °C. This specific allotropic carbon is also the hardest substance known, and it expands less on heating than any other material. These diamond properties are a logical consequence of the fact that carbon atom forms covalent bonds (sp³) to four neighboring carbon atoms arranged toward the corners of tetrahedron. The strength of the C-C bonds plus their arrangement in space provide the unusual properties of diamond. Diamond is an excellent insulator, opposite to graphite. The specific feature of amorphous carbons relates to the fact that they usually have unpaired electrons (dangling bonds).
 | Fig. 3 Diagram for the Z-DOL molecule adsorbed on the carbon surface at 20 °C (Yanagisawa, 1994; Bhushan and Zhao, 1999). |
Table 3 Functional Group to Total Carbon Ratio on Hydrogenated DLC Surfaces and Their ICN's | |||
| Functional Group | Formula | % Total Carbon | ICN |
| Ether | —C—O—C— | 15.7 | 20 |
| Carbonyl | ═C═O | 6.0 | 65 |
| Ester | —COOR | 3.5 | 60 |
| Carboxyl | —COOH | 0.5 | 150 |
| Hydroxyl | —COH | 0.3 | 100 |
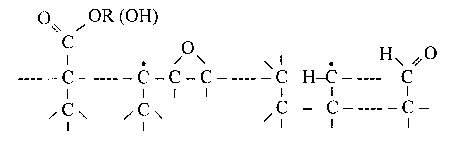 | Fig. 4 Simplified diagram of the chemical structure of a hydrogenated carbon overcoat surface. |
The ratio of oxygen-containing functional groups presented in Fig. 3 does not relate to their percentage of total carbon. Even considering inorganic character numbers (ICN), as discussed by Yanagisawa (1994), the most abundant functional groups would relate to the ether group (15.7% of total carbon with ICN = 20), and the carbonyl group (6.0% of total carbon and ICN = 65). The presence of other oxygenated groups (—COOR, —COOH, and —OH) is much less significant, although their ICN's are high, particularly for the carboxyl group (ICN =150). The inorganic character number shows the relative magnitude of association between function groups.
 | Fig. 5 Aromatic layer plane with functional side groups (Janzen, 1982). |
Hydrogen incorporated in the amorphous carbon changes its chemical and physical properties. For example, electrical resistance and hardness of the carbon film vary with the amount of hydrogen. A hydrogenated carbon surface chemistry differs significantly from a nonhydrogenated one (Wang et al., 1996). The same is true for the triboemission process (Nakayama, Bou-Said, et al., 1997). However, it is not due to dangling bonds. Yanagisawa(1994) discussed the question why dangling bonds are not saturated by hydrogen, referring to papers by Wada et al. (1980). The authors of the two latter papers emphasized that dangling bonds in carbon are relatively stable. Dangling bonds are not easy to bond to hydrogen because double bonds in sp² carbon would be saturated first with hydrogen (Cho et al., 1990). According to Jansen et al. (1985), even if the carbon is hydrogenated, the density of unpaired
 |
| Fig. 7 Concentration of carbonyl and hydroxyl end groups of a-C:H films (Wang et al., 1996). |
The emission intensities of the negatively charged particles and positively charged particles are low in that region of hydrogen content. Figure 8 also manifests that the charge intensity of negative particles is greater than that of positive particles, particularly in the low hydrogen content region. In the region of hydrogen contents between 15 at. % and 37 at. %, the emission intensity of the charged particles increased very rapidly, accompanied by the photon emission. It is of note at this point that the friction coefficient also increased from a low to a higher value in the region of hydrogen content from 15 to 27 at. %. The relationship of the friction coefficient values and hydrogen content in the carbon films tested is presented in Fig. 9. From these results, Nakayama, Bou-Said, et al. (1997) concluded that a microplasma state is formed at the frictional contacts of diamonds sliding on hydrogenated carbon films.
3.2.2. Interaction Mechanisms of a Carbon Overcoat with PFPE Lubricants
There is a wide variety of issues, combined with a possibility of obtaining adequate lubrication and protection of the disk surface. One is the PFPE lubricant thickness and its bondability with the carbon surface. A second is the chemical stability of the lubricant film under sliding conditions. Another issue is the ability of the lubricant to flow across the surface. While it must have an interaction with the DLC (a-C:H) overcoat that is strong enough to prevent loss during spinning, it must at the same time be able to flow into small regions of the surface that are depleted of lubricant as a result of contact with the head. Unhydrogenated amorphous carbon has a small energy gap and thus has a relatively high electrical conductivity. The small energy gap results possibly because of increased presence of sp² bonds. Resonance hybridization leads to delocalization of the electrons within a plane. Because electrons are free to move through the plane, they can conduct an electric current. Such material usually develops dangling bonds. Kaplan et al. (1985) found, however, that the hydrogenated amorphous carbon continues to have a loss energy gap of 1.2 eV in comparison with the direct gap of purely sp² bonded carbon (7.3 eV). This led to the conclusion that the presence of sp² bonding states requires the hydrogen not only to passify the dangling bond but also to saturate the double graphite bonds (sp²). Thus, for a-C:H films, it is of importance to know to what extent hydrogen passifies the dangling bonds and how these control the carbon surface activity and electrical conductivity. This issue also interrelates with electron emission intensity during sliding, as presented in Fig. 8. It is also pointed out that the electrical conductivity and hardness have been found to depend on the carbon preparation conditions (Enke, 1981; Cho et al., 1990). Amorphous hydrogenated carbon films of high electrical resistivity were studied by Miller and McKenzie (1983), using electron spin resonance both as prepared and after vacuum heat treatment. They found that all the carbon films investigated had a high spin density in the range from 10²¹ to 10²² spins/kg. Dangling bonds in a-C:H overcoats should be of particular importance from the viewpoint of the carbon overcoat interaction with PFPE lubricants. Most interestingly, for a-C:N materials, Torng and Silvnrtsen (1990) found that the energy gap is increased from 1.1 to 1.4 eV when the nitrogen partial pressure increased from 2.5 mTorr to 10 mTorr. These values are very close to the energy gap value (1.2 eV) for hydrogenated amorphous carbon (Kaplan et al., 1985). At the moment we have not found any information on triboemission from the a-C:N substrate; however, comparing the energy gap data for both a-C:H and a-C:N materials, we can speculate that nitrogenated carbon also emits electrons under boundary friction conditions, either in contact with diamond, alumina, or any DLC substrate. As with a 2.5-mTorr N2 partial pressure in the plasma, the nitrogen content in the carbon is already quite high (25 at. %; Torng and Silvertsen, 1990); we can assume that lower nitrogen content a-C:N substrates emit fewer electrons than a-C:H substrates. Considering the interaction of lubricants with surfaces of hydrogenated carbon films, we must bear in mind what types of active sites exist on the surface (see Figs. 3 and 4) and how they can interact with the lubricant molecule. Thus, the most important factor controlling this interaction will relate to the chemistry of the PFPE end groups. Accordingly, lubricants with polar end groups such as hydroxyl, carboyl, or piperonyl can strongly interact with the DLC surface, forming bonded films. To achieve necessary wear protection, the magnetic medium is covered with a film (10-20 nm; see Fig. 6 ) overcoat of a wear-resistant amorphous hydrogenated carbon, and it is coated with a molecularly thin film (1-2 nm) of a perfluoropolyether lubricant.- perfluorodiethyl ether,
- perfluoropentane,
- perfluoro-octane, 2,2,2-trifluoroethanol, and 1,1, 7-H-perfluoroheptanol.
4. DEGRADATION MECHANISMS OF PFPE LUBRICANTS
4.1. Introduction
A PFPE lubricant deposited on thin hard carbon coatings under static conditions interacts with the surfaces mostly through physical adsorption forces. The physically adsorbed lubricant film can, however, also be chemically bonded by a specific treatment of the deposited film. The known approaches to produce chemically bonded films encompass exposure of the lubricated disk to specific radiation modes, such as- low-energy X-ray (Heideman and Wirth, 1984),
- nitrogen plasma (Homola et al., 1990),
- high-energy ion beam (Lee, 1991), and
- low-energy electrons and far ultraviolet (Vurens et al., 1992).
- thermal decomposition processes,
- catalytic degradation processes,
- electron mediated degradation, and
- mechanical degradation by a shearing process.
4.2. Thermal Decomposition
PFPE's as a class of fluid lubricants exhibit excellent thermal and oxidative stability. Notwithstanding the fact that they are thermally stable up to 350 °C (Helmick and Jones, 1990), there is clear evidence that their actual thermal stability is adversely affected by the presence of metals. Jones et al. (1983) demonstrated the effects of steel and some titanium alloys and additives on the thermal stability of linear perfluoroalkyl ether fluids. The presence of metal alloys drastically increases the rate of degradation. Two inhibitors, a perfluoroalkyl ether substituted monophospha-s-triazine and a perfluorophenylphosphine, were found to be highly effective in degradation reduction at 288 °C, but they had only limited effectiveness at 316 °C. Results of another work (Zehe and Faut, 1989) showed that complete degradation of Fomblin Z takes place at 185 °C in the presence of iron oxide (Fe2O3).Interestingly, such degradation was not observed with the Krytox lubricant. The complete degradation of Fomblin Z was ascribed to the presence of acetal groups (—O—CF2—O—) in the polymeric chain, as suggested earlier by Jones et al. (1983).
 | Fig. 11. Thermal degradation process of PFPE lubricants (temp. >350 °C); the free radicals can react with PFPE molecules and oxygen molecules, recombine with other free radicals, or decompose. |
The presence of oxygen in the environment will lead to the thermal oxidative degradation process in which a very reactive peroxide radical can be generated.
4.3. Catalytic Degradation
As a-alumina exists as the structural element in the construction of a head slider for a magnetic disk drive, the degradation mechanism of PFPE's by a-alumina was examined (Kasai et al., 1991). The results obtained led to a followup experiment of the degradation process in the presence of AlCl3, and at higher temperatures (Kasai et al., 1991; Kasai, 1992). Although a-alumina is a typical Lewis acid, A1C13 is a stronger Lewis acid. According to Kasai (1999), the above-mentioned studies conclusively demonstrated that the degradation of PFPE's in the presence of Lewis acids (metal oxides or halides) is singularly dominated by the intramolecular disporportionation reaction,|
R1—CF2—O—CF2—O—CF2—R2 → R1—CF3 + FC(O)—R2 |
where R1 is the left part of Z-DOL molecule from the above-shown acetal sector, and R2 is the right portion of Z-DOL molecule.
- it is catalyzed by A1F3 formed during the induction period;
- it uniquely involves the acetal moiety (—O—CF2—O) of the polymer chains;
- it generates methoxy end groups (—O—CF3), and
- it results in fragmentation of polymer chains but does not involve an unzipping process.
 |
| Fig. 12. Interaction between a Lewis acid site on AlF3 and an acetal sector of Z-lube (Kasai et al., 1991). |