TRIBOCHEMISTRY
C. KAJDASWarsaw University of Technology, Institute of Chemistry in Plock, 09-400 Plock, Poland, e-mail: ckajdas @ zto.pw.plock.pl
SUMMARY
Chemical reactions of tribological additives proceeding during the boundary lubrication process involve the formation of a film on the contact surface and protect it during friction. Reactions initiated by mechanical action concern tribochemistry. Tribochemical reactions are distinct from those of thermochemical ones. The same is due to catalysis and tribocatalysis. The aim of this paper is to review and discuss tribological experimental results aiming at answering some specific questions. How far the triboemission process can influence tribochemistry of the tribological system? What is the part of the negative-ion-radical action mechanism (NIRAM) in initiating tribo-chemical reactions of metals and ceramics? What are their specific reactive intermediates? Can tribochemistry be interconnected with selected heterogeneous catalytic reaction processes ? Additional objective of the present paper is to attempt proving the hypothesis saying that specific intermediate reactive species for both tribochemical reactions and some heterogeneous catalytic reactions are formed by the same mechanism mostly governed by the NIRAM approach.Keywords: tribochemistry, boundary lubrication, lubrication mechanism, NIRAM, catalysis
INTRODUCTION
Lubricant components either adsorbed or chemisorbed on friction solid surfaces form a lubricant film that under boundary lubrication conditions can react with the surface or on the surface. Chemical reactions of anti-wear and extreme pressure additives, proceeding under boundary lubrication conditions generate a film reducing wear and damage of the contact solids. The application of mechanical energy associated with friction releases physical processes that can be the cause of tribochemical reactions of solids with lubricant molecules. Figure 1 demonstrates a general approach to physical and chemical events under boundary lubrication conditions. Chemical reactions initiated by mechanical action concern tribochemistry that deals with the chemical changes of both solids and lubricant molecules due to
 |
| Figure 1: Major physical and chemical events in the boundary lubrication contact |
the influence of boundary and/or mixed lubrication operating conditions. From the view-point of chemical reactions at surfaces, a very comprehensive review of mechanically initiated reactions is described in [1]. It is stressed that many tribological problems are concerned with various tribochemical reactions that cannot be accounted for by frictional heat alone. Another review [2] emphasizes the technological importance of the mechanical initiation of chemical reactions in such processes as grinding, drilling, crushing and cutting which are often facilitated by chemical compound formation at the worked interface. Tribochemical reactions are distinct from those of thermochemical ones because activation energy (Ea) for the latter is much higher. For example, Ea for iron oxide formation from iron and oxygen differs very significantly if the thermochemical reaction (Ea=54 kJ/mol) is compared with tribochemical reaction (Ea=0.7 kJ/mol) [1]. Similar dependence may be observed for other reactions. The occurrence of reaction velocity independent of temperature shows clearly that the energy for releasing the reactions is applied to the solid through the mechanically activated regions only. The same is due to catalysis and tribocatalysis. It is to note at this point that chemically stimulated exoelectron emission occurs continuosly from silver catalyst during partial oxidation of ethylene and the emission rate is proportional to the rate of ethylene oxide formation [3]. The central thrust of this paper is to review and discuss a wide variety of tribological experimental results relating to metals, ceramics as well as diamond-like carbon (DLC). It aims mostly at answering some specific questions concerning both tribochemistry and catalysis. Additional objective of the present paper is to look for links between tribochemistry and tribocatalysis in terms of the NIRAM concept.
2 BOUNDARY LUBRICATION AND TRIBOCHEMISTRY
An excellent and detailed review of the boundary lubrication history is described by Dowson [4]. Boundary lubrication is quite complex and even difficult to be clearly defined. However, it is an extremely interesting research area due to still existing many unknowns, even in respect to the anti-wear behaviour of fatty acids and/or tribochemistry of fatty alcohols and esters. Boundary lubrication is often described as a condition of lubrication, in which the friction and wear between two surfaces in relative motion are determined by the properties of the surfaces and by the properties of the lubricant other than viscosity. This definition is closely related to the Hardy's first approach to the boundary lubrication process [5]. Taking into account what Campbell [6] wrote 30 years ago, one can note that not too much better understanding of the boundary lubrication complexity was gained in this period. He emphasized that boundary lubrication is perhaps the most confusing and complex aspect of the subject of friction and wear prevention. This statement might consider the fact that what is known mostly relate to the friction coefficient.When oxides of the contact area are thick and porous they are ruptured easily. That results in both plastic deformation of the sliding surfaces and a wide variety of physical phenomena, particularly those related to the triboemission process. Disruption of the surface oxide layer generally results in some physical processes and usually in higher friction and severe wear. The wear debris produced are entrapped at the interface and plowing and microcutting of the surfaces occurs. During these interactions (a) forces are transmitted, (b) energy is consumed, (c) physical and chemical natures of these materials are changed, and (d) the surface topography is altered. Understanding the nature of these interactions is addressed to tribophysics [7] which describes the basic mechanisms that govern interfacial behavior and illustrate how the basic theories can be applied to provide practical solutions to important friction and wear problems. Physics and chemistry of tribological wear has been discussed in [8]. The formation of fresh surface sites forms a real bridge between physics and chemistry of the wear processes leading to microscopic aspects of a wide variety of wear mechanisms. These mechanisms are often connected with tribochemical reactions that are initiated by the surface enlargement effects. Typical physical phenomena evolved by wearing processes encompass mostly high temperature and triboemission. Detailed information concerning the triboemission process and related phenomena are described in [9]. Triboemission is defined as emission of electrons, charged particles, lattice components, photons, etc., under conditions of boundary friction conditions and/or surface damage caused by fracture processes. Figure 2 illustrates the triboemission process connected with the surface enlargement during friction.
 |
| Figure 2: Physical processes evolved by friction [9] |
One of the most important component of triboemitted particles encompasses electrons. Accordigly, the triboemission process is of particular significance for both the boundary friction process as such and tribochemistry of the boundary lubrication process.
3 BASIC INFROMATION ON THE NIRAM APPROACH
3.1 General Description
Under rubbing conditions two types of activated sites on friction surfaces can be created, that is, thermally activated sites and sites activated by the exoelectron emission (EEE) process. Exoelectrons are electrons of low-energy (0 - 4 eV) spontaneously emitted from most fresh surfaces, or they are electrons that are emitted from surface atoms under certain conditions that provide enough energy to provoke their evaporation. Apart from low-energy electrons other particle types, mostly, ions and lattice components, are also emitted from solids during rubbing process as depicted in Figure 2. The formation of negative ions and their decomposition process are simulated by electron attachment mass spectrography [10]. This type of mass spectrography uses electrons of an energy range similar to those of exoelectrons (1 - 4 eV). Accordingly, the energy of triboelectrons (exoelectrons) is sufficient to form negative ions or negative-ion-radicals of a wide variety of compounds which, in turn, as reactive intermediates, may easily react with the positively charged surface sites of the friction solid (metal, ceramic, DLC, composite) counterparts, yielding given chemical compounds. Figure 3 presents schematically the triboelectron emission process.
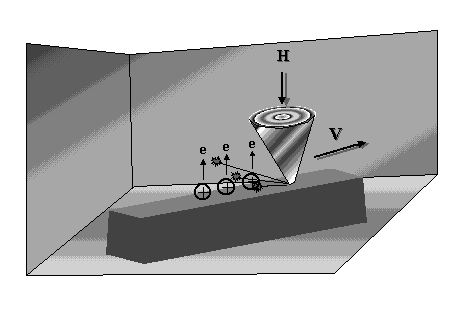 |
| Figure 3: Schematic of the electron emission process during rubbing |
Most recent research [11] provided clear evidence that the major part of negatively charged particles emitted from diamond, alumina, and sapphire is low-energy from zero to 5 eV. This finding allows to consider the NIRAM approach as an important factor initiating typical tribochemical reactions of the environment compounds with mechanically treated solids or more specifically, being under boundary lubrication conditions. Actually, the chemical reactions under friction conditions include several types of reaction processes (i) "pure" tribochemistry, i.e. specific chemical reactions initiated only by the mechanical action of the system (mechanochemistry), (ii) reactions of thermochemical processes (e.g., oxidation, degradation), and (iii) catalytic processes related to both catalysis and tribocatalysis. The specific tribochemical reactions proceed according to both the free radical mechanism and ionic mechanism. The shearing process generates free radicals and other tribochemical reactions comprise ionic and free radical reaction types. Negative-ion-radical intermediate species are of particular interest. Therefore, some 15 years ago the negative-ion-radical action mechanism (NIRAM) approach was proposed [12-14]. Recently, this concept has been reviewed and its importance for lubricant compound reactions with the friction surfaces was presented [15]. In brief, the NIRAM approach considers the following major phases:
- Low-energy electron emission process and creation of positively-charged spots, generally on tops of asperities;
- Action of the emitted electrons with the lubricant causing the formation of reactive intermediates -- negative ions and free radicals -- on the rubbing surfaces;
- Reaction of negative ions with metal surface, and other reactions, e.g., free radical reactions, forming mostly an organometallic film, which protects the rubbing surfaces from wear;
- If shear strength is high, it can cause cracking of chemical bonds producing inorganic films and further radicals.
- Eventual destruction of the wear protecting layer, followed by the electron emission process and subsequent formation of a new protective film according to stages (a) through (d).
| A + :B ® A:B |
The species A:B is called a coordination compound or an acid-base complex. Since the HSAB principle is a pragmatic one and has its application as a predictive and correlative principle it seems to be important to apply this approach for getting a better understanding of lubrication mechanisms.
3.2 Example of Specific Reactive Intermediates
This example considers reactive intermediates generated by low-energy electrons and UV irradiation. In work [17] it has been hypothesized that low-energy electron mediated degradation of PFPE lubricants under sliding conditions of computer head on hard disk in UHVC is the dominant mechanism. The hypothesis was very helpful in better understanding and accounting for all the major experimental findings. Another work [18] demonstrated the negative ions emitted from the Demnum S200 lubricant during irradiation with low-energy electrons (less than 14 eV). They found that the most intense fragment ion relates to F- at m/e = 19. This is not unexpected since F- is a very stable ion. Figure 4 presents the discussed mass spectrum. Other ions that of strong intense are C3F5O2- and C3F7O - at m/e = 163 and m/e = 185, respectively. The negative ion (m/e) - = 185 is generated via C-O bonding cleavage in one end of the Demnum S200 molecule. However, the mechanism by which ion (m/e) - = 163 is formed is unknown. Most recent paper [19] aimed at accounting for this specific ion formation mechanism.The detailed Z-DOL lubricant degradation mechanism, as described in [17], emphasizes the importance of the
 |
| Figure 4: Negative ions emitted from the Demnum S200 lubricant during irradiation with low-energy electrons [18] |
negative-ion-radical action mechanism for the PFPE lubricant degradation process. Interestingly, the NIRAM approach can also be applied in accounting for experimental results concerning chemical bonding of PFPE lubricant films with DLC under sliding conditions. Earlier paper [20] demonstrated how low-energy electrons may attach to the PFPE lubricant molecules and allow the chemical reactions between lubricant molecules and a DLC surface. All the discussed results clearly suggest that anionic intermediates play an important role in the electron-induced degradation process of PFPE lubricants. Review paper [21] discusses and analyses the literature concerning the interaction and degradation mechanisms of perfluoropolyether lubricants with carbon protective overcoats used for magnetic media.
Formation mechanism of the negative species (m/e) - = 163 generated from the Demnum S200 lubricant can be interpreted as follows. After splitting off the (m/e) - = 185 anion (CF3CF2CF2O-) from the Demnum S200 molecule, the left free radical oO-CF2-CF2-CF2-O-CF2- CF2-CF2 ....... interacting with a low-energy electron can form the following negative-ion-radical species:
| oO-CF2-CF2-CF2-O- |
with the m/e ratio of 182. This reactive intermediate splits off one free radical (Fo) generating the negative ion (m/e) - = 163 having the following structure:
| O=CF-CF2-CF2-O- |
which corresponds to the strong signal found in the negative-ion mass spectrum of the Demnum S200 lubricant (Figure 4). Although the catalytic degradation mechanism is widely accepted in the literature -- basing on a wide variety of discussed experimental data -- it is reasonable to emphasize that anionic intermediates (negative ions and/or negative-ion-radical species) produced by low-energy electrons play an important part in the electron mediated degradation process of PFPE lubricants. This is in line with the negative-ion-radical action mechanism (NIRAM) approach. This approach is evidenced somehow by elucidating the formation mechanism of the negative ion (m/e) = 163 from the negative-ion-radical species ?(m/e).- = 182.
4 TRIBOCHEMISTRY OF ALIPHATIC HYDROCARBONS
4.1 General Remarks
Important compounds of mineral base oils and some synthetic ones, e.g., poly-a-olefin oils, relate to aliphatic hydrocarbons. Hexadecane (n-C16H34), also known as cetane, is widely used as the low-viscosity model base oil to investigate both tribological effectiveness and tribochemical reaction mechanisms of various antiwear and extreme pressure additives, as well as friction modifiers. It is a non-polar compound and thus does not compete with tribological additives on the surface adsorption process. Generally, it is accepted that freshly purified hexadecane is far poorer lubricant than material which has stood for some time, particularly in a transparent glass bottle. Early work on chemistry of boundary lubrication of steel by hydrocarbons demonstrated that the sliding behaviour of steel lubricated by hydrocarbons under boundary lubrication conditions could be related to chemical reactions at the sliding surfaces involving metal, hydrocarbon, and oxygen. The results suggested that the reactions occur at sites where fresh metal surface was exposed by rubbing. Most recent study [22] clearly demonstrates that the oxidation process under friction conditions is very specific and the major oxidation compounds from n-hexadecane under boundary friction conditions relate to other oxygenates than carboxylic acids. Mechanism by which tribochemical reactions of hexadecane proceed under boundary lubrication conditions has not been clearly established yet.4.2 Reaction Mechanism
To obtain negative ions of paraffins, HO- ions have to be attached [10]. This mechanism of ionization can be tested with small amount of heavy water vapour in the ion source. Another mechanism of ionization [(M - 1) -]
 |
| Figure 5: Fragmentation of (CnH2n+2.OH) - ions |
was found in work [25]. Under the conditions of the investigation only (M - 1) - ions were formed by n-hexane molecules. The stability of (M + HO-) ions decreased with a decrease in the molecular weight of the n-paraffins. The intensity of M+17 peaks increase from n-heptane to n-nonane in the ratio 1:2.5:6.
It is to note that major ions formed closely relate to those that are generated from alcohols, as described in [24]. This clearly evidences the similarity of FTIR spectra taken from wear scars lubricated with alcohols and those taken from wear scars generated under boundary friction conditions lubricated with n-hexadecane.
5 TRIBOCHEMISTRY OF ESTERS
It is well known that esters under boundary lubrication conditions form soap with the friction metal surface. The lubrication related literature suggests that the soap formation by the ester under boundary lubrication is due to the hydrolysis process of the ester. This leads to the formation of a very small amount of the fatty acid which when adsorbed attach the metal to form the corresponding soap film. On the other hand, it is supposed that the soap formation proceeds according to the NIRAM approach. It is assumed that the soap formation is due to the ester C-O bond cleavage leading to the formation of the RCOO- species which reacts with the positively charged surface spot to form the soap. Recent study [26] aimed at elucidating the actual reaction mechanism by which a series of palmitic esters, used as antiwear additives to n-hexadecane, reduce the wear of the steel-on-steel system. It has been assumed [27] that alcohols are physically adsorbed on the reactive metal surfaces; esters with reactive metals such as zinc and cadmium, however, cause chemical attack. It was emphasised that the reaction with the ester is due to hydrolysis. The hydrolysis reaction is known to be catalysed by acids or bases. A carboxylic ester is hydrolysed to a carboxylic acid and on alcohol with aqueous acid or aqueous base. Base promotes hydrolysis of esters by providing the strongly nucleophilic reagent OH-. It is self evident that under alkaline conditions the carboxylic acid is obtained as its salt, for example the sodium salt, from which it can be liberated by addition of mineral acid. The alkali hydrolised reaction is essentially irreversible due to a resonance - stabilized carboxylate anion which shows little tendency to react with an alcohol. On the other hand, acidic hydrolysis is reversible,
RCOOR1 + H2O | ¬H+® | RCOOH + R1OH |
thus the mechanism for acidic hydrolysis is also the mechanism for esterefication. Mineral acid speeds up both processes by promoting carbonyl oxygen and this rendering carbonyl carbon move susceptible to nucleophilic attack. In hydrolysis, the nucleophile is a water molecule and leaving group is an alcohol; in esterifcation, the roles are exactly reversed. Based on the above information it is difficult to realise that under typical boundary lubrication conditions mineral acid might be present to catalyse the ester hydrolysis process. To check this we have carried out a set of experiments aiming at finding if typical conditions existing under boundary lubrication could cause the hydrolysis process. Under there conditions, but without a catalyst, we have not found any evidence for the acid formation from aliphatic esters. Experiments performed in that work were designed in a way aiming at checking what might be the effect of a possible breaking of the ester bond by hydrolysis on the lubricant anti-wear property. Thus, two series of lubricants have been selected. The first lubricant series includes n-hexadecane with ester additives at the concentrations ranging from 0,03% to 1,5% wt. The second series of lubricants relates to mixtures of these ester substrates (acid and alcohol), added to n-hexadecane at the same concentrations as for the esters. Equimolar ratio of the acid to alcohol was used for all the mixtures applied. If under the testing conditions the ester hydrolysis actually involves the breaking of the ester bond to produce an acid and an alcohol, the anti-wear behaviour of the esters should correspond to the anti-wear behaviour of their substrates. In other words, the equimolar acid/alcohol mixture at very low concentration in n-hexadecane should not provide higher relative wear reduction of the ball than the ester at much higher concentration. Figure 6 shows examples of the ball wear reduction versus the additive concentration in n - hexadecane.
The most striking finding, clearly demonstrated in Figure 6, relates to the fact that the acid/alcohol mixtures investigated have the same effect on the steel-on-steel wear process. Their anti-wear property does not change with the concentration in n-hexadecane. Additionally, the mixtures affect dramatically the wear reduction of steel under boundary lubrication conditions. Results obtained in this work enable to state that the hypothesis saying "during the friction process under boundary lubrication conditions, lubricated by aliphatic esters, the ester hydrolysis process cannot proceed without an adequate catalyst" was proved for aliphatic esters dissolved in n-hexadecane.
Another interesting finding relates to the best wear reduction ability of n-hexadecyl palmitate and the equimolar mixture of palmitic acid and 1-hexadecanol
 |
| Figure 6: Influence of the extreme concentrations (300 ppm and 1.5%) of two palmitates and equimolar mixture of palmitic acid and C14 alcohol in n-hexadecane on the pin-on-disk ball wear [28] |
[27]. This finding is interpreted in terms of the chain matching effect with n-hexadecane. More research is needed to clarify these interactions.
6 TRIBOCHEMISTRY OF CERAMICS
6.1 Silicon Nitride and Silicon Carbide
As a possible application of the NIRAM - HSAB approach for lubrication of ceramics, detailed account for tribochemical wear reactions of silicon nitride with water and alcohols was recently discussed in a review paper on tribochemistry of ceramics [28]. Usually, tribochemical reaction between silicon nitride and water to form silica is postulated via termochemical reaction process as follows:
| Si3N4 + 6H2O ® 3SiO2 + 4NH3 | (1) |
| SiO2 + 2H2O ® Si(OH)4 | (2) |
 |
| Figure 7: Relationship between moles of water adsorbed on silicon nitride powder and moles of ammonia evolved under grinding conditions [29] |
The ratio of gases evolved ngas (in moles) to moles of the water vapour adsorbed nads, is around 0.11. On the other hand, the average ratio ngas/nads for Equation (1) is 0.666 which is several times higher than the ratio determined experimentally. The difference can be explained in terms of the NIRAM-HSAB approach as presented in Figure 8.
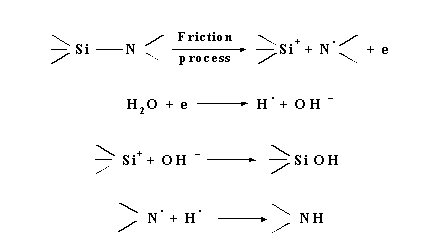 |
| Figure 8: NIRAM - HSAB Model for reaction of water with silicon nitride under friction conditions [28] |
Very similar approach seems to be applicable to silicon carbide. The suggested tribochemical reaction process of silicon carbide with water under rubbing conditions is depicted in Figure 9.
 |
| Figure 9: NIRAM - HSAB Model for reaction of water with silicon carbide under friction conditions |
6.2. Tribochemistry of Alumina and Silica
The well known fact of hydroxide formation from alumina under friction conditions in the presence of water molecules can also be explained in terms of the NIRAM - HSAB approach. Under rubbing conditions the emitted electrons produce OH- and H• species from water and positively charged sites ( Al+ ) and free radicals ( Al-O• ) from the alumina substrate. HSAB interaction and radical recombination produce the hydroxyl groups (Al-OH ). Similar tribochemical process can be considered for silica. Figure 10 depicts that situation.
 |
| Figure 10: NIRAM - HSAB Model for reaction of water with silica under friction conditions |
7 LINKS BETWEEN TRIBOCHEMISTRY AND CATALYSIS
Numerous chemical reactions relating also to catalytic processes can proceed with the same velocity at significantly reduced temperatures in comparison to the thermal reference procedure [1]. This situation is very similar to the non-catalytic thermochemical reactions and their tribochemical analogues. Therefore from the view-point of the reaction mechanism both catalytic processes and typical tribochemical reactions might relate to the same driving force -- governed by the affect of exoelectrons. Heterogeneous catalytic processes can be initiated by thermally emitted electrons. Tribocatalytic process is the typical catalytic reaction enhanced by the action of triboelectrons produced in the test system. This hypothesis can be evidenced by earlier experiments described in [3]. That work studied electron emission from silver catalyst during partial oxidation of ethylene by measuring simultaneously the emission rate and the rate of the ethylen oxide formation. Fig. 11 demostrates the effect of temperature on the emission rate of exoelectrons and on the formation rate of ethylen oxide, for which the temperature of the silver catalyst was raised step-wise from 25-210°C followed by the descent to 25°C.
 |
| Figure 11: Effect of temperature on the exoelectron emission rate and the formation rate of ethylene oxide [3] |
8 CONCLUSIONS
The present paper is an interdisciplinary review of extremely fast developing research on tribochemistry. This review demonstrates the importance of a pathway combining the negative-ion-radical action mechanism (NIRAM) approach with the Hard and Soft Acids and Bases (HSAB) concept in initiating tribochemical reactions. Major features of the lubrication model which presents a specific reaction cycle of lubricant components with friction solid contact spots is depicted in Figure 12.
 |
| Figure 12: Reaction cycle of lubricant components on solid contacts during friction |
The most important conclusion is that the NIRAM approach is associated with both tribochemistry and heterogeneous catalysis.Characteristic IR common feature of n-hexadecane reflects in very specific absorption bands of wear products appearing at around 1550 cm -1, 1650 cm -1 and 3300 cm -1 for different metals. Similar IR spectra relate also to tribochemical products of alcohols and esters. These specific spectra are also typical for both tribochemistry of ethers [31] and tribochemistry of fatty acids [32]. Finaly, it seems that the most recent research [33] provides a good deal of additional evidence for the importance of NIRAM approach in initiating tribochemical reactions.
9 REFERENCES
- [1]
- G. Heinicke: Tribochemistry. Akademie Verlag, Berlin 1984
- [2]
- Fox P.G.: Review. Mechanically Initiated Reactions in Solids. J. Mat. Sci., 10 (1975) 340-360
- [3]
- Sato N., Seo M.:Chemically Stimulated Exoelectron Emission from Silver Catalyst During Partial Oxidation of Ethylene. J. Catal., 24 (1972) 224-232
- [4]
- D. Dowson: History of Tribology. Longman, London 1979
- [5]
- W. Hardy: Collected Works. Cambridge University Press 1936
- [6]
- Campbell, W.F: Boundary Lubrication. In: Boundary Lubrication. An Appraisal of World Literature. New York: ASME 1999, 87-113
- [7]
- N.P. Suh: Tribophysics. Prentice-Hall, Egelwood Cliffs, New Jersey 1986
- [8]
- Kajdas, C.: Physics and Chemistry of the Wear Process. In: Proceedings of 10th International Tribology Colloquium. Ostfildern: TA Esslingen 1996, 37-62
- [9]
- Kajdas, C.: Furey, M.J.: Ritter, A.L.: Molina, G.J.: Triboemission as a Basic Part of the Boundary Friction Regime. Lubr. Sci., accepted for publication
- [10]
- M. von Ardenne: Steinfelder, K.: Tuemmler, R.: EA-Massenspektrographie organischer Substanzen. Springer Verlag, Berlin 1971
- [11]
- Molina, G.J.: Furey, M.J.: Ritter, A.L.: Kajdas, C.: Triboemission from Alumina, Single Crystal Sapphire, and Aluminum, Wear, 249, (2001) 3-4, 214-219
- [12]
- Kajdas, C.: On a Negative-Ion Concept of EP Action of Organo-Sulfur Compounds. ASLE Trans., 28 (1983) 21-30
- [13]
- Kajdas, C.: About a Negative-ion Concept of the Antiwear an Antiseizure Action of Hydrocarbons During Friction. Wear, 101 (1985), 1-12
- [14]
- Kajdas, C.: About an Ionic-radical Concept of the Lubrication Mechanism of Alcohols, Wear, 116 (1987), 167-180
- [15]
- Kajdas, C.: Importance of Anionic Reactive Intermediates for Lubricant Component Reactions with Friction Surfaces. Lubr. Sci., 6 (1994) 3, 203-228
- [16]
- Kajdas, C.: A Novel Approach to Tribochemical Reactions. Generalized NIRAM - Hard and Soft Acids and Bases Action Mechanism. In: Tribochemistry. Proceedings of ITC Yokohama 1995, Forum on Tribochemistry. JST, Tokyo (1995) 31-35
- [17]
- Zhao, X.: Bhushan, B.: Kajdas, C.: Lubrication Studies of Head-disk Interfaces in a Controlled Environment. Part 2. Degradation Mechanisms of Perfluoropolyether Lubricants. Proc. Inst. Mech. Engrs., Part J, 214 (2000) 547-559
- [18]
- Vurens, G.H.: Gudeman, C.S.: Lin, L.J.: Foster, J.S.: Mechanism of Ultraviolet and Electron Bonding of PFPEs. Langmuir 8 (1992) 1165-1169
- [19]
- Kajdas, C.: Major Tribochemical Reaction Pathways Governing the Nanotribochemistry Processes. Proc. ITC Conference, Nagasaki 2000, Satelite Forum Tribochemistry. Tsukuba 2000, 57-62
- [20]
- Zhao, Z.: Bhushan, B.: Kajdas, C.: Tribological Performance of PFPE and X-1P Lubricants at Head-Disk Interface. Part II. Mechanisms. Tribol. Lett. 6 (1999) 141-148
- [21]
- Kajdas, C.: Bhushan, B: Mechanism of Interaction and Degradation of PFPEs with a DLC Coating in Thin-Film Magnetic Rigid Disks: A Critical Review. J. Info. Storage Proc. Syst., 1 (1999) 303-320
- [22]
- Kajdas, C.: Makowska, M.: Gradkowski, M.: Tribochemistry of Hexadecane under Boundary Lubrication Conditions. Proc. Nordtrib Conf. Porvoo, Finland, VTT, Espoo 2000, 594-603
- [23]
- Kajdas, C.: Al-Nozili, M.: Wear Behaviour and Tribochemistry of Oxygenates in 1-Methylnaphyhalene under Boundary Lubrication of the Steel-on-steel System. Tribologia, 30 (1999) 301-314
- [24]
- Kajdas, C.: Obadi, M.: Wear Behaviour and Tribochemical Reactions of Low Molecular alcohols under Boundary Lubrication of the Steel-on-Aluminium System. Tribologia, 30, (1999) 273-87.
- [25]
- Kajdas, C.: Negative Ionisation of n-Paraffins C6-C9, C20, C22 and C24 in EA-Mass Spectrograph (in Polish). Rocz. Chem.., 45 (1971) 1771-1775
- [26]
- Kajdas, C.: Shuga`a, K.: The Investigation of Antiwear Properties and Tribochemical Reactions of Palmitates in the Steel-on-Steel System. Tribologia, 29 (1998) 389-402
- [27]
- Bowden, F.P.: Moore. A.C.: Physical and Chemical Adsorption of Long-Chain Compounds on Metals. Research, 2 (1949) 585-586
- [28]
- Kajdas, C.: Tribochemistry of Ceramics. Tribologia, 2 (1998) 148-167
- [29]
- Volante, M.: Fubini, B.: Giamello, E.: Bolis, V.: J. Reactivity Induced by Grinding in Silicon Nitride. Mat. Sci. Letters, 8 (1989) 1076-1078
- [30]
- Mizuhuara, K.: Hsu, S.M.: Tribochemical Reaction of Oxygen and Water on Silicon Surfaces. In: Wear Particles. Elsevier Publishers B.V. 1992, 323-328
- [31]
- Molenda, J.: Kajdas, C.: Makowska, M.: Gradkowski, M.: Unsaturated Oxugen Compounds as Antiwear Additives for Lubricants. Tribologia, 3 (1999) 323-331
- [32]
- Kajdas, C.: Majzner, M.: Tribological Behaviour of Fatty Acids under Boundary Lubrication Conditions .(in Polish). Tribologia (Wkladka), 1 (2000) 195-203
- [33]
- Hsu, S.M.: Zhang, J.: Yin, Z.: Shen, M.C.: Zhang, X.: Tribochemistry Induced by Nano-Mechanical Scratches. Proc. ITC Nagasaki 2000 Tribochemistry, Tsukuba 2000, 25-30